Acute rejection grade
A0
No rejection
A1
Minimal rejection
A2
Mild rejection
A3
Moderate rejection
A4
Severe rejection
Airway inflammation grade
B0
No significant inflammation
B1R
Low-grade inflammation
B2R
High-grade inflammation
BX
Ungradeable
Chronic airway rejection grade
C0
Not present
C1
Present
3.1.1 Acute Rejection
3.1.1.1 Grade A0
No rejection; there are no significant perivascular inflammatory cell infiltrates. The presence of a few small noncircumferential infiltrates is not sufficient for a diagnosis of A1 (Figs. 3.1 and 3.2).
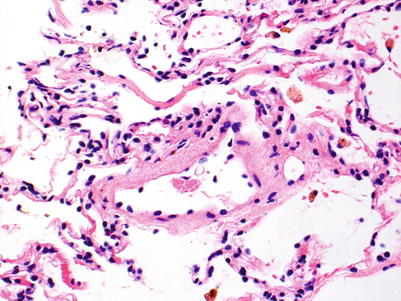
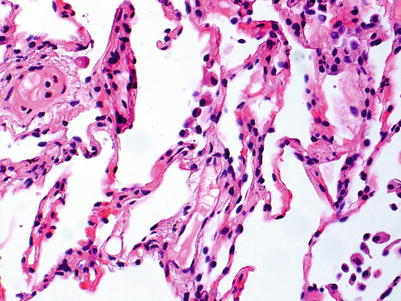

Fig. 3.1
Postcapillary venule with few scattered mononuclear cells, primarily lymphocytes, in adjacent interstitium. The sparse inflammatory cell infiltrate is not diagnostic of acute rejection

Fig. 3.2
Small postcapillary venule with few scattered neutrophils in nearby capillaries. This biopsy is 3 weeks post-transplant, and the mild neutrophilic infiltrate is normal in this setting
3.1.1.2 Grade A1
Minimal rejection; there are scattered and infrequent perivascular mononuclear cell infiltrates in alveolar tissue with only few eosinophils and no endotheliitis. The presence of endotheliitis would indicate at least grade A2 rejection. Some (if not all) infiltrates should be entirely circumferential around vessels. If many vessels are involved, this may not be “minimal” rejection and we tend to regard this as grade A2 (Figs. 3.3 and 3.4).
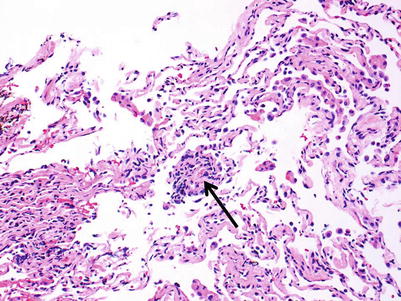
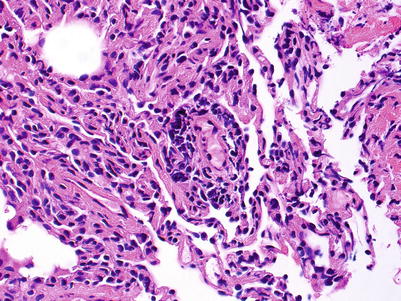

Fig. 3.3
Small lymphoid infiltrate centered on a postcapillary venule (arrow). Note the lumen containing red cells in the center of the picture. The alveolar tissue is mildly collapsed but the small perivascular infiltrate is distinct and entirely circumferential, indicating grade A1. There are no identifiable endotheliitis, eosinophils, or interstitial inflammation in alveolar walls

Fig. 3.4
Distinct lymphoid infiltrate centered on a postcapillary venule, consistent with grade A1. Despite the slight crush artifact, it is easy to see there are no identifiable endotheliitis, eosinophils, or interstitial inflammation in alveolar walls
3.1.1.3 Grade A2
Mild rejection; this grade is characterized by more frequent and larger perivascular chronic inflammatory cell infiltrates around venules and arterioles, often with accompanying eosinophils and endotheliitis. Venules appear to be more frequently involved than arterioles. The presence of only rare eosinophils is not sufficient to warrant a diagnosis of A2 rejection by itself, but it should prompt careful review of the biopsy material for more features of grade A2. Furthermore, the absence of endotheliitis does not exclude grade A2 rejection if other features are present. There is obviously room for interpretive differences in frequency and size of perivascular inflammatory cell infiltrates between grades A1 and A2; the ISHLT guidelines suggest the perivascular infiltrates of A2 are more easily recognizable at lower scanning power. This is fair advice, but experienced pathologists are able to detect small A1 perivascular infiltrates at low power and must be able to adjust their grading criteria accordingly (Figs. 3.5 and 3.6).
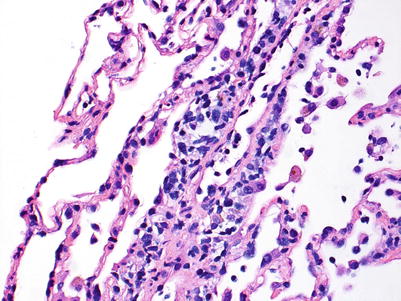
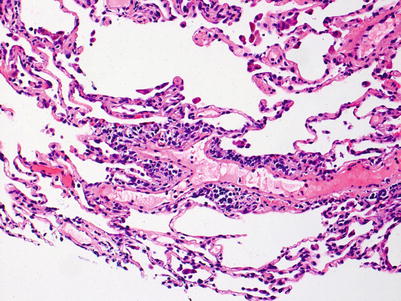

Fig. 3.5
Prominent mononuclear cell infiltrate around a venule and accompanying endothelial inflammation, consistent with grade A2. There are scattered inflammatory cells in nearby alveolar septa but there is no obvious expansion of the interstitial space

Fig. 3.6
Prominent mononuclear cell infiltrate around a venule and scattered eosinophils, consistent with grade A2. Similar to Fig. 3.5, there are scattered inflammatory cells in nearby alveolar septa but there is no obvious expansion of the interstitial space
3.1.1.4 Grade A3
Moderate rejection; this grade is defined by the extension of the inflammatory cell infiltrate into the alveolar septa. There are usually concurrent endotheliitis and frequent eosinophils but not always. There may be features of mild acute lung injury with fibrin deposition but there are no hyaline membranes (Figs. 3.7, 3.8, and 3.9).
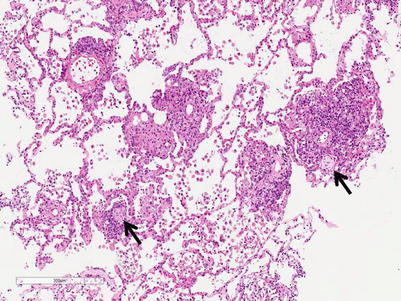
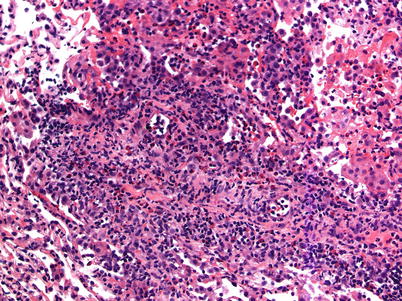
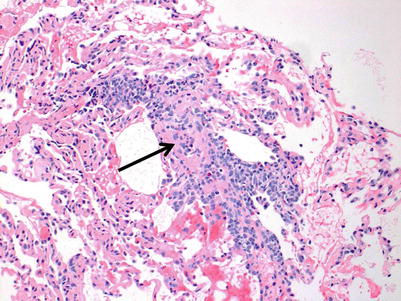

Fig. 3.7
Prominent mononuclear cell infiltrates around multiple vessels with interstitial inflammation and few small polyps of organizing pneumonia (arrows), consistent with grade A3

Fig. 3.8
Very prominent mononuclear cell infiltrate around a vessel with mixed interstitial inflammation, including eosinophils, and expansion from a case with grade A3

Fig. 3.9
Prominent mononuclear cell infiltrate around a venule with endotheliitis, few conspicuous eosinophils, and interstitial inflammation and expansion in adjacent alveolar septa, consistent with grade A3. The endotheliitis and intravascular inflammation is so prominent, it is difficult to discern the lumen of the vessel in the center of the picture (arrow)
3.1.1.5 Grade A4
Severe rejection: this grade of rejection is characterized by concurrent severe acute lung injury with features of acute cellular rejection, specifically perivascular and interstitial mononuclear cell infiltrates with or without endotheliitis and eosinophils. The acute lung injury demonstrates hyaline membranes and reactive pneumocyte injury. Paradoxically, the rejection-associated inflammatory cell infiltrates may be diminished, compared with grade A3 or A2 rejection, and distinction from nonrejection causes of lung injury may be difficult. Pathological confirmation of perivascular infiltrates and exclusion of infection by special stains and/or microbiology studies, and clinical correlation are usually sufficient to confirm the diagnosis of severe rejection (Figs. 3.10, 3.11, and 3.12).
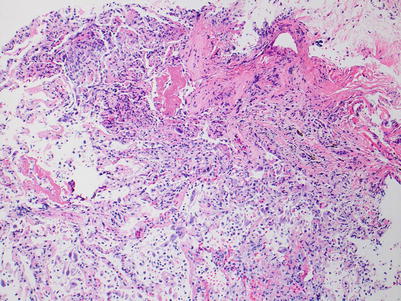
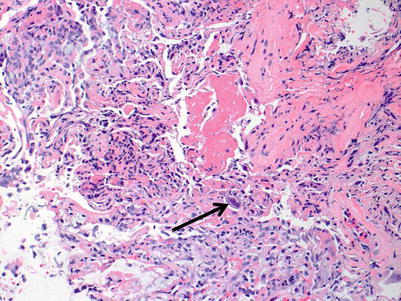
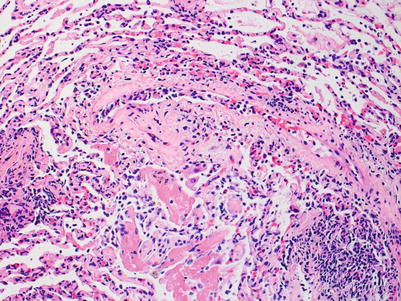

Fig. 3.10
Diffuse acute lung injury with fibrin deposition, interstitial and alveolar cellular infiltrates, and pneumocyte injury. Obvious biopsy distortion is appreciable at the top of the image; nevertheless, the severe and diffuse acute lung injury is readily apparent. The etiology of the acute lung injury is not obvious in this field of view

Fig. 3.11
Higher-power image of Fig. 3.10 reveals reactive pneumocyte changes within the severe acute lung injury. The large reactive nucleus with a purple hue (arrow) may raise concern for viral inclusion. However, this reactive pneumocyte with nucleomegaly is a mimicker for viral infection. Viral studies on this biopsy were negative; the patient had missed antirejection medications for several weeks and was clinically more likely to have rejection than infection. The etiology of the acute lung injury is still not apparent in this field of view

Fig. 3.12
On this area of the same biopsy from Figs. 3.10 and 3.11, a vessel with clear endotheliitis and mild perivascular and adjacent interstitial cellular infiltration is seen. These features are diagnostic of rejection. In the lower portion of the image, acute lung injury with fibrin deposition is seen; this corresponds to grade A4 rejection. Note the relative diminution of the cellular infiltrate in comparison with the images of A3 rejection. This case serves to demonstrate the importance of sufficient sampling, careful pathological review of the entire specimen, and clinical correlation before diagnosis is rendered
3.1.2 Airway Inflammation
The previous edition of the ISHLT lung transplant grading system divided airway inflammation into 5 grades, from B0 (no inflammation) to B4 (severe inflammation) [3]. To simplify the system and improve reproducibility among pathologists, the most recent ISHLT grading system collapsed the grades into 3 categories: B0 (no significant airway inflammation), B1R (low-grade bronchiolar inflammation), and B2R (high-grade bronchiolar inflammation). Grade B1R is a combination of previous grades B1 and B2; and grade B2R is a combination of previous grades B3 and B4. “R” is used to designate the revision and to avoid confusion with the previous grades. Grade BX is used to designate uninterpretable airways if the airways are not sampled, are distorted by crush or sectioning artifact, or if there is a concurrent infection causing airway inflammation.
3.1.2.1 Grade B0
No significant inflammation; there may be scattered chronic inflammatory cells associated with the small airways but the infiltrate would be within normal limits.
3.1.2.2 Grade B1R
Low-grade bronchiolar inflammation; there are varying degrees of mononuclear cells within the submucosa and muscular layers of the small airway. There may be mild lymphocytic infiltration of the epithelium but there is no significant epithelial cell injury. Small vessels around the airway may have circumferential perivascular infiltrates suggestive of acute rejection grade A1. If these vessels are part of the airway, the inflammation should be considered a component of the airway inflammation and not acute rejection (Figs. 3.13 and 3.14).
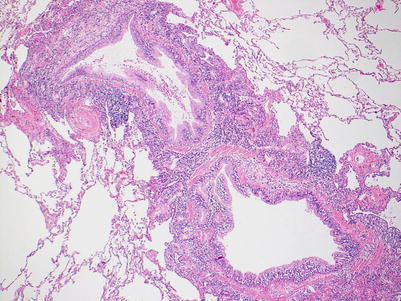
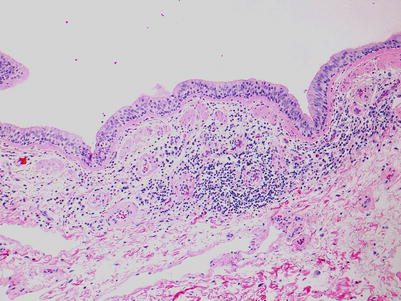

Fig. 3.13
Low-power view of a bronchiole with prominent circumferential mononuclear cell infiltrate including cellular infiltration within the deeper epithelium. There is no appreciable epithelial cell injury and this is most consistent with B1R

Fig. 3.14
The bronchiolar wall has a conspicuous mononuclear cell infiltrate in the deeper connective tissue. Note the involvement and inflammation of small vessels in the wall. There is mononuclear cell infiltration within the epithelium, but overall, the infiltrate is mild and the paucity of epithelial cell injury is most consistent with B1R
3.1.2.3 Grade B2R
High grade; this grade is characterized by more intense intraepithelial lymphocytic infiltration and epithelial cell injury manifesting as metaplasia or frank necrosis. Eosinophils are more common than in grade B1R. If there are abundant neutrophils, an infectious etiology should be considered and, if proven or favored, grade BX should be rendered (Figs. 3.15 and 3.16).
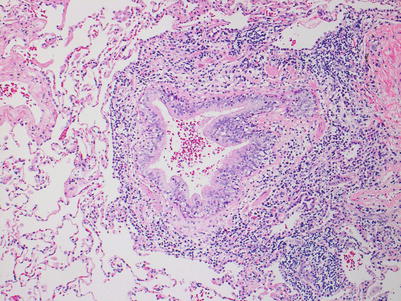
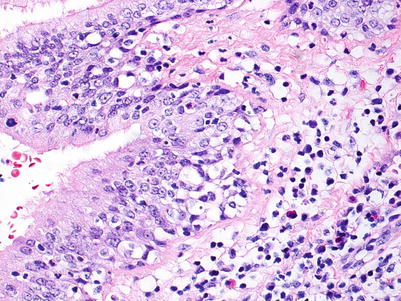

Fig. 3.15
This bronchiole shows very prominent circumferential mononuclear cell infiltration including cellular infiltration throughout the entire epithelium. There is disruption of the normal epithelial architecture with epithelial cell dehiscence in the areas of most prominent inflammation. Overall, the features are most consistent with B2R

Fig. 3.16
High-power view of Fig. 3.15 in an area of epithelial inflammation and injury. Note the mixed cellular infiltrate including occasional neutrophils and eosinophils and the loss of normal epithelial architecture near the basement membrane. In this case, intact cilia may be seen in some areas; this does not preclude the presence of significant epithelial cell injury in deeper portions of the epithelium
3.1.2.4 Grade BX
Ungradeable; when no airway is present on the biopsy or is obscured by handling artifact or infection-associated inflammation, this is designated as grade BX. Data from the Banff Study of Pathologic Findings Associated with HLA Antibodies found 38 % of cases, from a pool of 253 biopsies, were graded BX [4].
3.2 Chronic Rejection/Chronic Lung Allograft Dysfunction
Chronic rejection of the lung allograft is the major cause of graft failure and death in lung transplant patients. Classically, chronic rejection has been recognized as the development of an obstructive defect termed bronchiolitis obliterans syndrome (BOS). As understanding and experience of chronic lung transplant rejection has increased, various phenotypes have been recognized and the term chronic lung allograft dysfunction (CLAD) has been coined to accommodate all forms of chronic allograft rejection in the lung [5]. All forms of CLAD are poorly demonstrated on transbronchial biopsies and are best evaluated with clinical and radiographic evaluation.
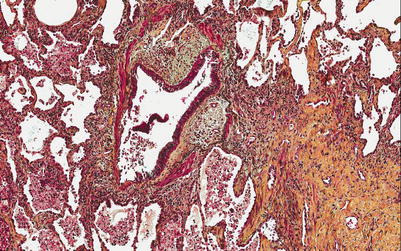
The pathological features are usually best seen on explant or autopsy specimens. Gross evaluation of explant specimens reveals fibrous adhesions to the chest wall or between lobes. On cross section, the pleura and interlobular septa are more prominent and fibrotic (Figs. 3.17 and 3.18).
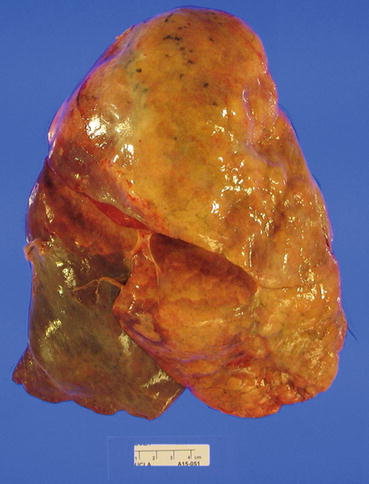
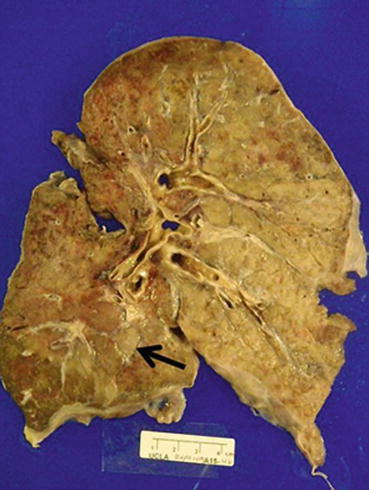

Fig. 3.17
External surface of a transplanted right lung at the time of autopsy. Note the adhesions between the middle and the upper lobes (Courtesy of Dr. Josephine Aguilar-Jakthong)

Fig. 3.18
Cross-section of the right lung allograft. Note the subtle accentuation of interlobular septa and pleural thickening, consistent with septal (arrow) and pleural fibrosis (Courtesy of Dr. Josephine Aguilar-Jakthong)
3.2.1 Bronchiolitis Obliterans
BOS manifests as a decrease in forced expiratory volume per 1 second (FEV1), may result in eventual graft loss and contributes to the 5-year graft survival of approximately 50 % [6]. The development of BOS is not reliably evaluated by the transbronchial biopsy and is best monitored by clinical and radiological evaluation. Nevertheless, the presence of airway scarring, termed bronchiolitis obliterans (BO), should be reported if discovered on a transbronchial biopsy. If there is no evidence of airway scarring, the grade is C0; if scarring is present, the grade is C1. The scarring begins as subepithelial granulation tissue with small vessels within an edematous fibrous stroma. The process may be concentric or eccentric. Eventually, the granulation tissue is replaced by dense fibrous scar tissue that causes progressive obstruction of the small airways and may ultimately lead to complete obliteration. The findings may be difficult to appreciate on a hematoxylin and eosin (H&E) stain and the features can be highlighted with special connective tissue stains, such as Movat, Masson trichrome, or elastic stains (Figs. 3.19 and 3.20).
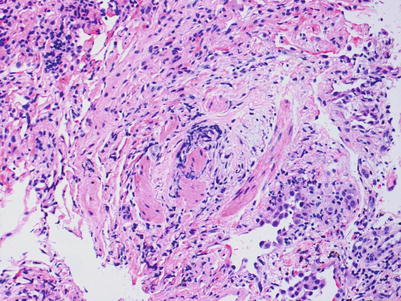
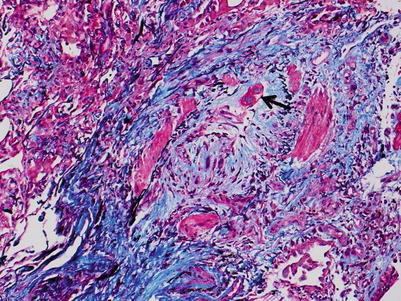

Fig. 3.19
Area of the bronchiolar wall with smooth muscle bundles, fibroconnective tissue, and mild nonspecific chronic inflammation. It is difficult to discern which part of the bronchiolar wall is pictured. There is no identifiable lumen and the smooth muscle bundles may be from an area of tangential sectioning. The rounding of the bundles of smooth muscle may be suggestive of an obliterated airway, but the diagnosis is difficult to confirm with this image

Fig. 3.20
Same area of bronchiolar wall from Fig. 3.19. Note the well-defined elastic layer within the smooth muscle bundles and the reactive fibrosis obliterating the airway. This is diagnostic of Bronchiolitis Obliterans (BO), or grade C1. Note the residual airway epithelium (arrow). This image demonstrates the value of special connective tissue stains (Combined Masson trichrome and elastic van Gieson stain)
Distal to the lesion, postobstructive changes, such as clusters of foam cells or cholesterol clefts with granulomas, may be seen (Fig. 3.21). However, these are nonspecific findings and, although these features are suggestive, do not indicate a diagnosis of BO without sampling of the lesion in the airway. Pathological features of BO are more easily discovered in larger resection specimens such as allograft explants (Figs. 3.22 and 3.23).