Fig. 4.1
Renal tissue core obtained with a 16-gauge needle contains several blood-filled glomeruli, which appear as congested bulging hemispheres (arrows). Sclerotic and inflamed glomeruli with little or no circulating blood are notoriously difficult to identify. Medullary tissue (not shown) is easy to recognize because it displays streaks or parallel lines corresponding to tubules and has no glomeruli
4.1.2 Specimen Processing
4.1.2.1 Light Microscopy
The Banff 97 recommendation [3] for specimen processing is as follows: at least seven slides with multiple sequential 3- to 4-μm sections should be cut. Three of these slides should be stained with hematoxylin and eosin (H&E) stain, three with periodic acid–Schiff (PAS) stain or silver stains, and one with a trichrome stain. At UCLA, eight sequential sections of 3-μm thickness are cut. Levels 1–4 are stained sequentially as follows: H&E, trichrome, PAS, and Jones methenamine silver (JMS) stain. Levels 5–8 are stained in a similar fashion. The turnaround time for these studies is usually less than 24 h from time of biopsy. We routinely process H&E-stained sections for same-day analysis when clinically indicated. For STAT cases, H&E-stained slides can be obtained within 4 h, thus allowing for preliminary evaluation of acute cellular rejection, acute tubular injury/necrosis (ATI/ATN), the presence or absence of thrombosis, and estimation of tubulointerstitial scarring. For more detailed assessment, special stains are needed.
4.1.2.2 Immunofluorescence Microscopy
The primary role of IF in the evaluation of the renal transplant is to assess for C4d deposition in antibody-mediated rejection (AMR). Therefore, C4d immunofluorescence studies are required in every case. In addition, IF studies are helpful in identification of recurrent and de novo glomerular disease. IF has little role in the diagnosis of T-cell–mediated rejection except to rule out other differential diagnoses. A full IF panel (including immunoglobulin G [IgG], IgA, IgM, C3, C1q, fibrinogen, and kappa and lambda light chains) is generally required only on samples where glomerular disease is suspected. On all transplant cases, the minimal IF panel for our group includes C4d and fibrinogen (to aid in the assessment of thrombotic microangiopathy—discussed later).
4.1.2.3 Electron Microscopy
Gluteraldehyde-fixed tissue is embedded into epoxy resin and 1-μm sections are cut. Survey (“thick”) sections are stained with toluidine blue to evaluate the tissue and assess for the presence of glomeruli by LM prior to EM analysis. In some cases, these sections may contain diagnostic material not otherwise seen in the tissue for LM. Like IF, EM has a more limited role in the transplant biopsy compared with native kidney biopsies. EM is most useful for evaluating features of chronic antibody-mediated rejection (CAMR; see later) and cases with suspected recurrent or de novo glomerular disease. EM is of no practical use in the diagnosis of T-cell–mediated rejection. EM need not be performed on every biopsy. In general, EM should be performed when glomerular disease is suspected. At UCLA, we perform EM in every new transplant case, in patients without prior biopsies over the past 6 months, and as clinically or pathologically indicated.
4.2 Pathological Classification of Diseases of the Renal Allograft and the Banff Classification
Renal allografts are targets for (1) alloreactive immune response (rejection) including T-cell–mediated rejection and AMR; (2) pathology related to the transplant procedure such as ATI/ATN due to prolonged ischemia time, vessel thrombosis, and ureteral obstruction; (3) medication-induced toxicity or disease susceptibility (immunosuppression) including calcineurin inhibitor (CNI) toxicity, PVN, and post-transplant lymphoproliferative disorder (PTLD); and (4) diseases that affect native kidneys, which may represent recurrent or de novo disease.
The differential diagnosis for allograft dysfunction changes over time. Within the first 6 months of transplantation (particularly <3 months), the primary differential diagnosis of allograft dysfunction includes acute rejection, acute ischemic injury, acute CNI toxicity, and acute pyelonephritis.
In allografts greater than 6 months old, the primary differential diagnosis of dysfunction includes CAMR, chronic CNI toxicity, hypertension, chronic obstruction/reflux nephropathy, chronic pyelonephritis, PVN, glomerular disease (recurrent or de novo), PTLD, and chronic changes of unclear etiology.
4.2.1 Banff Criteria
The most widely used scheme for the grading and reporting of kidney transplant pathology is the Banff classification [5]. Since its inception in 1993, the classification system has been continually revised and is still being refined [5]. The use of Banff criteria in grading kidney transplant rejection allows a measure of standardization for the improvement of diagnostic consensus, publication, and research. Furthermore, the Banff grading is based primarily on LM features that can be identified and graded and subsequently synthesized into a classification of rejection. The Banff classification uses six major diagnostic categories for renal allograft biopsies (Table 4.1) [6].
1. Normal | No allograft pathology |
2. Antibody-mediated changes | Owing to documentation of circulating anti-DSAs, and C4d, or allograft pathology (may coincide with categories 3, 4, 5, and 6) |
3. Borderline changes | Suspicious for acute T-cell–mediated rejection (may coincide with categories 2, 5, and 6) |
4. T-cell–mediated rejection | May coincide with categories 2, 5, and 6 |
5. sInterstitial fibrosis and TA | No evidence of any specific etiology |
6. Other | Changes not considered to be due to rejection, acute or chronic, may include isolated g, cg, or cv lesions (may coincide with categories 2, 3, 4, and 5) |
4.3 Acute Allograft Rejection
4.3.1 Acute T-Cell–Mediated Rejection
4.3.1.1 Clinical Presentation
Acute T-cell–mediated rejection (acute cellular rejection [ACR]) is most common in the first few months following transplant; however, it can occur at any time post transplant [7]. Severe ACR classically presents as an acute increase in creatinine with decreased urine output, weight gain, fever, and graft tenderness and swelling. Low-grade ACR can be clinically silent and appear as “smoldering ACR” on a protocol biopsy. However, there is great variability in the clinical presentations of all grades of ACR. Hematuria and proteinuria are uncommon manifestations of ACR.
4.3.1.2 Microscopic Features
The most common morphological features of ACR are interstitial inflammation and tubulitis. In severe cases, endarteritis may be observed.
Interstitium
T-cells and macrophages are the predominant cells in the infiltrate, but variable numbers of plasma cells, neutrophils, and eosinophils may also be seen. Interstitial edema is an accompanying feature in the great majority of cases (Fig. 4.2). The inflammation is cortical and patchy; however, in severe cases, spillover into the medulla can be seen. Occasional, marked plasma cell accumulation is observed—so-called “plasma cell–rich” ACR (Fig. 4.3). The differential diagnosis for plasma cell–rich infiltrates in a renal allograft includes PVN and post-transplant lymphoproliferative disorder (PTLD); therefore, these considerations need to be ruled out. Prominent neutrophilic infiltration should raise suspicion for acute AMR, particularly if neutrophils are within peritubular capillaries (PTCs), or acute pyelonephritis, if accompanied by neutrophil casts in tubules (Fig. 4.4).
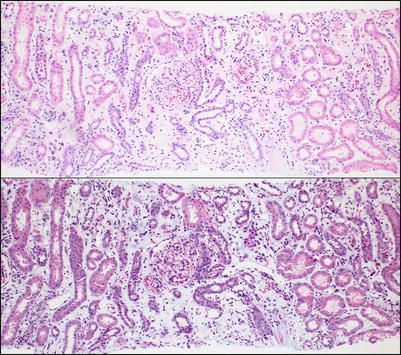
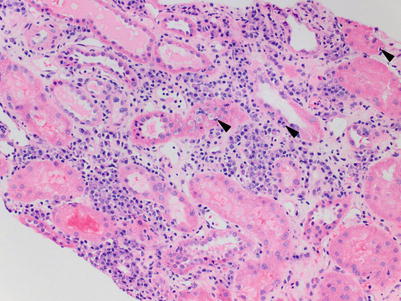
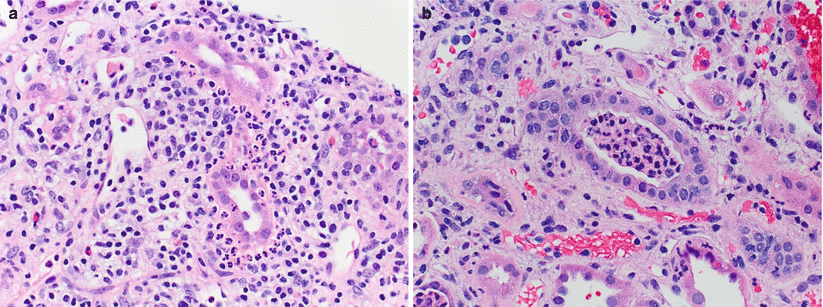

Fig. 4.2
Low-power view of the renal cortex shows diffuse interstitial inflammation associated with marked separation of the tubules as a result of interstitial edema, confirmed with the trichrome stain (bottom). Interstitial edema is a common accompanying feature of active interstitial inflammatory processes of any kind, including ACR

Fig. 4.3
There is a dense infiltrate primarily composed of clusters of plasma cells, associated with several foci of lymphocytic tubulitis (arrowheads). The differential diagnosis includes PTLD, PVN, and plasma cell-rich ACR. There are no reliable histological features to distinguish between these possibilities, and therefore, additional work-up with IHC stains is required

Fig. 4.4
(a) Interstitial inflammation with numerous neutrophils is not a typical feature of ACR and instead should raise suspicion for acute pyelonephritis (H&E, 200×). (b) The presence of intraluminal accumulations of neutrophils (neutrophilic casts) such as this one in a collecting duct represents strong evidence for a diagnosis of acute pyelonephritis and should warrant correlation with urine studies including cultures (H&E; 400×)
The Banff score for interstitial inflammation (i) is based on percentage involvement of nonscarred cortical parenchyma. However, data are emerging suggesting that even inflammation in scarred cortex is significant [8, 9]. Therefore, two interstitial inflammation scoring systems have been proposed: (i) percentage of inflammation in the nonatrophic cortex (Table 4.2) and (ti) total cortical inflammation. In the current classification system, a diagnosis of ACR requires inflammation involving at least 25 % of the nonscarred cortex (i2).
Banff score | Percentage of nonscarred cortex inflamed |
|---|---|
i0 | <10 |
i1 | 10–25 |
i2 | 26–50 |
i3 | >50 |
Tubules
The key morphological feature of ACR is tubule-infiltrating lymphocytes—tubulitis—which is best appreciated on PAS-stained sections where the tubular basement membrane can be visualized. Tubule-infiltrating lymphocytes appear as small, dark, basally located nuclei compared with the larger, pale, more apically located epithelial cell nuclei (Fig. 4.5). Tubulitis in areas of tubular atrophy (TA) is not currently considered a manifestation of ACR. The Banff tubulitis score (t) is based on number of mononuclear cells per transverse tubule cross section (or per 10 epithelial nuclei in longitudinal cuts) (Table 4.3).
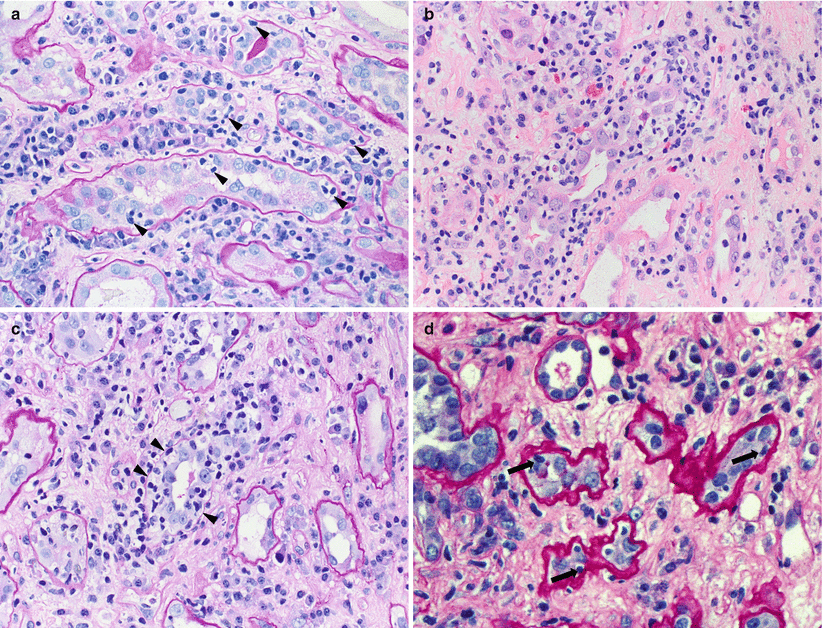

Fig. 4.5
(a) Several tubules show foci of tubulitis (arrowheads). Lymphocytes are smaller and have a darker nucleus compared with the tubular epithelial cells and often exhibit a “halo.” The tubular epithelial cells are displaced apically and exhibit reactive nuclear changes including enlargement and prominent nucleoli. There is an associated inflammatory cell infiltrate in the interstitium that includes activated lymphocytes (lymphoblasts), plasma cells, and monocytes (PAS stain). (b) In cases with severe tubulointerstitial ACR, the lymphocytes may obscure tubular structures, making it hard to identify foci of tubulitis. (c) The PAS stain is helpful in highlighting the residual basement membrane (arrowheads), thus facilitating visualization of the same tubule. In examples with extensive disruption of basement membranes, focal granulomatous inflammation may occur (not shown). (d) Atrophic tubules appear shrunken and demonstrate wrinkling and thickening of the basement membranes. Lymphocytic infiltration of atrophic tubules (arrows) is nonspecific and not diagnostic of ACR. However, if accompanied by tubulitis in nonscarred tubules, it may be indicative of a subacute cellular rejection process (PAS stain)
Banff score | Mononuclear cells/tubule |
|---|---|
t0 | 0 |
t1 | 1–4 |
t2 | 5–10 |
t3 | >10 |
Glomeruli
Glomeruli are usually spared in cases of tubulointerstitial ACR. However, glomerulitis, defined as the presence of glomerular intracapillary mononuclear inflammatory cell infiltration and endothelial cell swelling resulting in narrowing/occlusion of at least one capillary lumen, is occasionally observed in cases of ACR in the absence of acute AMR. The glomerulitis of ACR consists of T-cell glomerular infiltrates (versus macrophage infiltrates in AMR) [10]. However, glomerulitis is not included as a criterion of ACR (see Sect. 4.4).
Vessels
Vascular rejection may be a manifestation of severe ACR. Morphologically, this form of ACR is characterized by the presence of endarteritis (also known as intimal arteritis or endothelialitis), which predominantly affects arcuate and interlobular caliber arterial vessels, although arterioles may also be involved. The endothelial cells of the affected arteries appear “reactive” with increased basophilic cytoplasm and lifting from the underlying arterial media. The presence of subintimal infiltrating mononuclear inflammatory cells is the key diagnostic feature (Fig. 4.6). A single involved artery is sufficient for the diagnosis. Adherent, luminal mononuclear cells (Fig. 4.7) are not diagnostic of endarteritis, but warrant careful examination—including obtaining additional levels—because this finding is commonly associated with endarteritis. Endarteritis seen only at the biopsy edges should also be interpreted with caution, and in this setting, a diagnosis of suspicious for ACR vascular rejection may be most appropriate. In severe cases of vascular rejection, transmural inflammation and fibrinoid necrosis of arteries is observed. The Banff classification scores arteritis (v) based on the degree of luminal narrowing as a result of endarteritis in the most affected artery (Table 4.4). Arteries showing transmural inflammation and/or fibrinoid necrosis are automatically scored as v3.
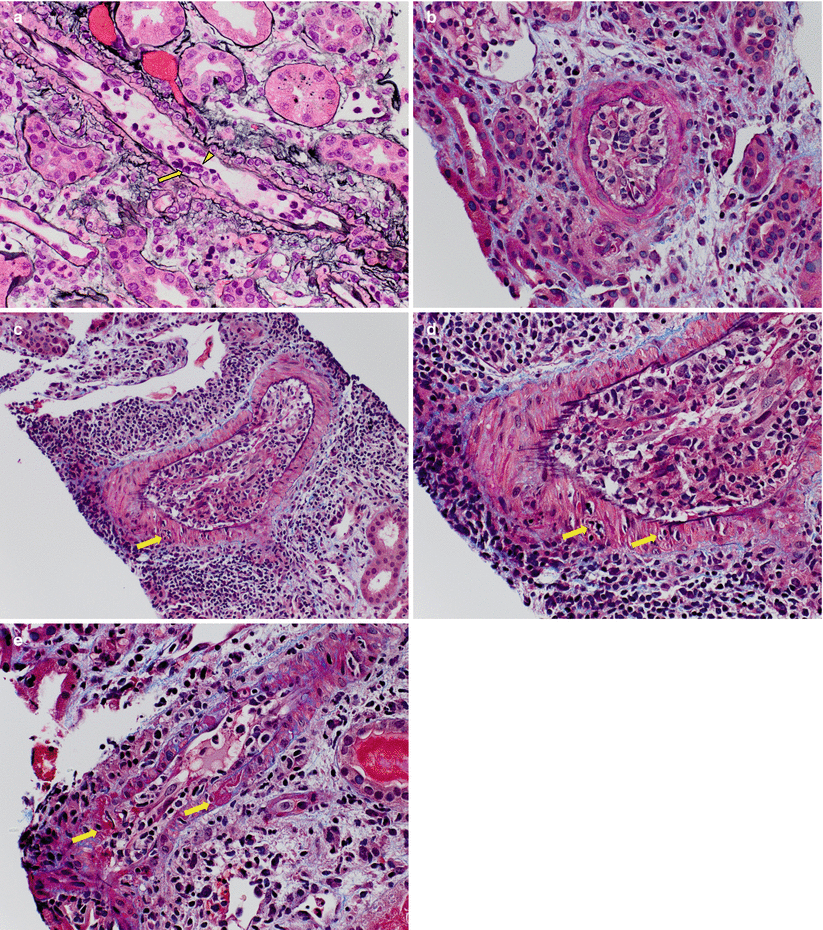
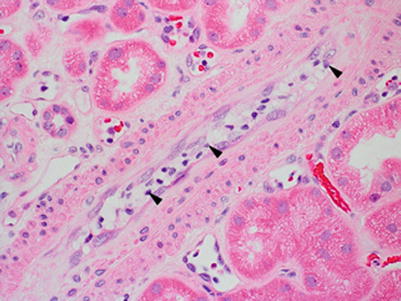

Fig. 4.6
(a) A small artery with endarteritis characterized by focal lymphocytic infiltration of the intima (arrows) causing lifting of the overlying endothelial cells (arrowhead) and mild narrowing of the lumen (Banff v1 score). The endothelial cells appear plump and prominent and there are several adherent leukocytes in the lumen (Jones methenamine silver stain). (b) A Banff v2 lesion involving a small artery, characterized by severe endarteritis leading to complete luminal occlusion (Trichrome–elastic Van Gieson [EVG] stain). (c) An interlobular artery shows severe endarteritis. In addition, there is focal transmural inflammation (arrow), thus categorizing this as a Banff v3 lesion (Trichrome-EVG stain). (d) On higher magnification, lymphocytes are seen infiltrating both the smooth muscle cell layer (arrows) and the lumen (Trichrome-EVG stain). (e) An example of fibrinoid necrosis involving the wall of a small artery (Banff v3 lesion). Trichrome stains are particularly useful in recognizing areas of fibrinoid necrosis, which appear as accumulations of bright red granular material corresponding to fibrin (Trichrome stain)

Fig. 4.7
An artery with plump endothelial cells and several marginated and adherent leukocytes (arrowheads); however, no lymphocytes infiltrating the intima are seen. Although this is not diagnostic of vascular rejection, it should prompt careful search for endarteritis because both typically coexist
Banff score | % of luminal area lost as a result of endarteritis |
|---|---|
v0 | 0 |
v1 | <25 |
v2 | ≥25 |
v3 | Transmural inflammation or fibrinoid necrosis |
4.3.1.3 Immunofluorescence Microscopy
IF is of limited practical use in the diagnosis of ACR. Fibrinoid necrosis is highlighted by staining for fibrinogen. C4d staining of PTCs would indicate concomitant AMR. Focal, granular staining of tubular basement membranes for IgG, C3, and C4d is seen in a small subset of cases of PVN (see later).
4.3.1.4 Classification of ACR
Banff classifies ACR into three grades (Table 4.5). Grade I ACR consists of greater than 25 % interstitial inflammation with moderate or severe tubulitis. Any vascular rejection is automatically classified as at least ACR grade II, regardless of tubulointerstitial inflammation.
Grade I | >25 % interstitial inflammation (i2, i3) | A | With moderate tubulitis (t2) |
B | With severe tubulitis (t3) | ||
Grade II | Endarteritis | A | Mild to moderate (v1) |
B | Severe (v2) | ||
Grade III | Transmural arteritis or fibrinoid necrosis (v3) | ||
Borderline | OR | Any tubulitis (>t0) with <25 % interstitial inflammation (≤i1) |
>25 % interstitial inflammation (i2,i3) with mild tubulitis (t1) |
4.3.1.5 Borderline/Suspicious for ACR
This category is used in cases with tubulointerstitial inflammation or tubulitis that do not meet criteria for a diagnosis of ACR. Borderline changes are observed in approximately 20 % of protocol biopsies 1 year post transplant [11]. Interestingly, there is poor intra- and interobserver reproducibility of this diagnostic category. In addition, management of patients with borderline lesions varies among centers. Besides early rejection, the differential diagnosis for borderline lesions includes other causes of low-level inflammatory cell infiltrate such as ATI/ATN, CNI toxicity, viral infection, and drug-induced hypersensitivity reactions.
4.3.1.6 Treated/Resolving ACR
Resolving/treated ACR is characterized by tubulitis out of proportion to the interstitial inflammation, sometimes associated with early interstitial fibrosis and TA (Fig. 4.8). Correlation with prior biopsies and clinical history is essential for this diagnosis.
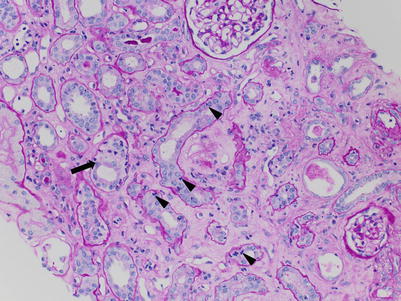

Fig. 4.8
Tubulitis out of proportion to interstitial inflammation sometimes accompanied by mild tubulointerstitial scarring is commonly seen in biopsies from patients who have received treatment for ACR. In this example, there are frequent and prominent foci of tubulitis involving mildly atrophic tubules (arrowheads) as well as nonatrophic tubules (arrow). There is only a sparse interstitial inflammatory cell infiltrate. This patient had been diagnosed with ACR type IB on the prior biopsy a few weeks earlier and had received immunosuppression treatment (PAS stain)
4.4 Acute Antibody-Mediated Rejection
4.4.1 Clinical Presentation
Occurs in patients who develop de novo donor-specific antibodies (DSAs) after transplant or those patients with preexisting DSAs from presensitization (blood transfusion, pregnancy, prior transplant). Acute antibody-mediated rejection (AAMR) is most common up to 3 weeks post transplantation, but may occur at any time (de novo DSAs usually take approximately 2 weeks to form, so very early AAMR would be due to preformed antibodies). AAMR typically presents with acute oliguric renal failure often requiring dialysis. The overall frequency of AAMR following transplant is 6 %, but it ranges from 8 to 43 % in presensitized patients [12]. One study found that AAMR criteria are met in nearly 24 % of biopsies for acute rejection [13].
4.4.2 Microscopic Features
Morphological manifestations of AAMR include (1) ATI without other apparent cause, (2) capillary inflammation (peritubular capillaritis, glomerulitis), (3) acute thrombotic microangiopathy (TMA), and (4) intimal or transmural arteritis. These may occur alone or in combination.
The histological hallmark of AAMR is microvascular injury in the form of glomerulitis and peritubular capillaritis. PTCs are often dilated and show increased leukocytes, including neutrophils and/or monocytes/macrophages (Fig. 4.9). Glomerulitis is a common finding in AAMR. As with peritubular capillaritis, the leukocytes may consist of mononuclear cells or neutrophils (compared with glomerulitis in ACR, which is T-cell predominant) (Fig. 4.10). Glomeruli may also show features of acute TMA including reactive-appearing endothelial cells, fibrin thrombi, and dissolution of the mesangium (mesangiolysis) (Fig. 4.11).
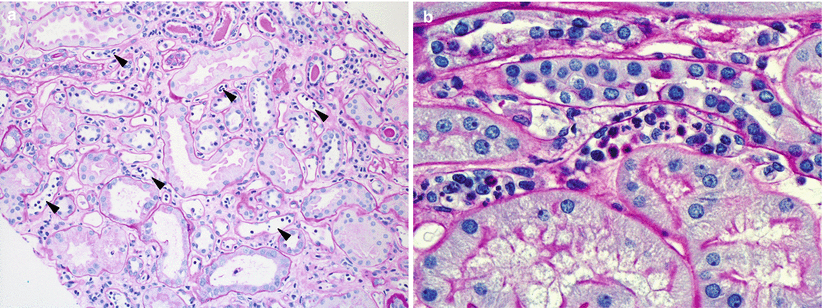
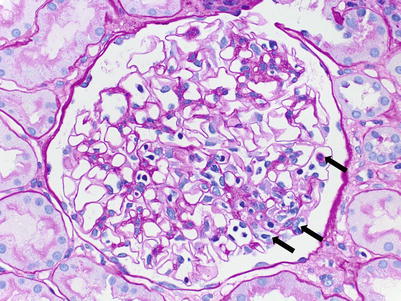
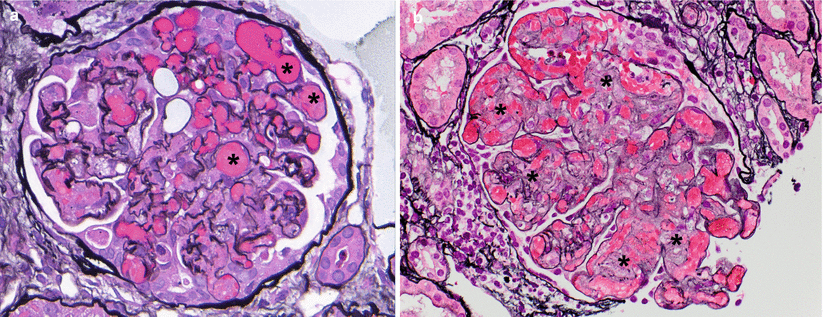

Fig. 4.9
(a) PTCs, best seen in PAS-stained sections, appear distended and contain increased numbers of circulating leukocytes. In the absence of significant interstitial inflammation as in this case, this finding is more specific for AAMR. (b) In most cases, intracapillary leukocytes comprise a mixed population including mononuclear cells and neutrophils. Occasionally, neutrophils can predominate

Fig. 4.10
A glomerulus shows intraluminal mononuclear leukocytes associated with endothelial cell swelling in capillary loops in a segmental distribution (arrowheads). Mononuclear infiltrates may represent ACR or AMR. The presence of neutrophils (not shown) would favor AMR over ACR (PAS; 200×)

Fig. 4.11
Features of acute TMA are variable, and morphology cannot reliably distinguish among the different causes. In the transplant setting, AAMR and CNI toxicity are the two most common etiologies. (a) Fibrin thrombi occluding several glomerular capillary lumens (asterisks) can be the dominant finding as in this case (JMS). (b) Mesangiolysis or dissolution of the mesangial matrix such as seen in this glomerulus can be another morphological feature of TMA, and it results in a fibrillary appearance of affected mesangial regions (asterisks) (JMS)
To be diagnostically significant, peritubular capillaritis has to be present in at least 10 % of the PTCs in the cortex. Banff PTC inflammation grading (ptc score) depends on the average number of intraluminal leukocytes and ranges from ptc0 to ptc3. Areas affected by acute pyelonephritis or necrosis and the subcapsular cortex should not be considered. Caution should also be taken not to score inflammatory cells in the medullary vasa recta. Banff glomerulitis is scored based on the percentage of glomeruli involved from g0 to g3 (Table 4.6).
ATI/ATN is present in 75 % of AAMR biopsies [14]. In our experience, ATI/ATN is too nonspecific to be considered a feature of AAMR, unless it is accompanied by at least mild interstitial inflammation and/or at least minimal peritubular capillaritis. The presence of significant mononuclear tubulitis should be interpreted as concurrent ACR and is reported in 30–80 % of AAMR biopsies [14, 15]. Very mild tubulitis is a common attendant finding in ATI/ATN and, therefore, should not be regarded as a manifestation of concomitant ACR; however, it can make the distinction from “borderline ACR” difficult.
There are no specific interstitial changes in AAMR. Interstitial edema with minimal/mild mononuclear inflammation (not diagnostic for ACR) may be present in pure AAMR. Interstitial hemorrhage and cortical infarction may be seen in patients with severe arterial involvement or TMA.
As with ACR, arterial injury in AAMR ranges from mild and limited to the intima (endarteritis) to severe with fibrinoid necrosis and minimal mononuclear cell infiltrate or transmural arteritis. A TMA pattern of vascular injury with mucoid intimal thickening and erythrocyte fragments may be present; however, arterial thrombosis is unusual.
4.4.3 Immunofluorescence Microscopy
The classic hallmark of AMR is C4d deposition in PTCs of the cortex and medulla. The fluorescence should be linear, circumferential, and crisp. C4d staining is often seen in glomeruli and arteries; however, these staining patterns are not specific for AMR but serve as internal controls (Fig. 4.12).
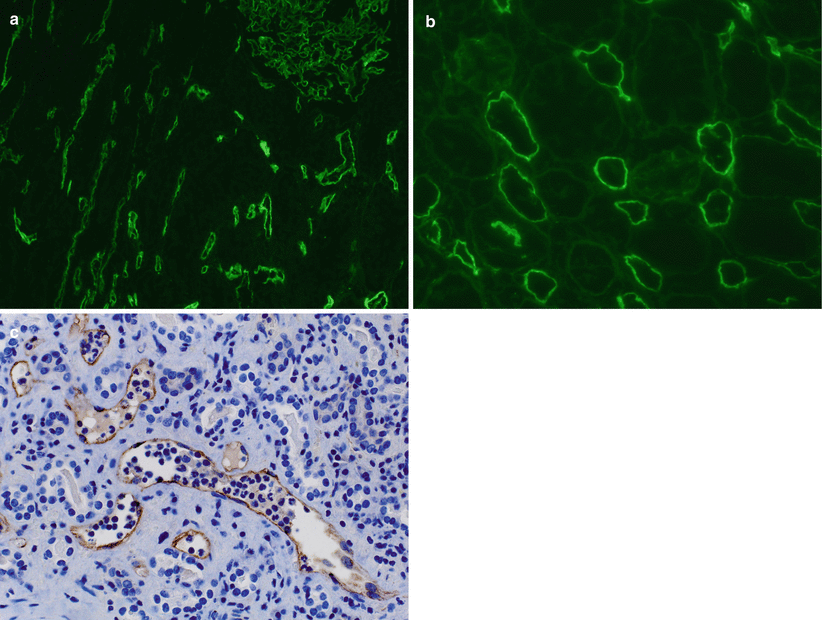

Fig. 4.12




(a) This IF image shows C4d staining of both a glomerulus (upper right corner) and PTCs. Glomerular and arterial (not shown) staining is a ubiquitous phenomenon and does not have clinical significance. Only staining of PTC is a criterion for the diagnosis of AMR. (b) C4d staining should be circumferential and strong, as in this example. (c) C4d detection by IHC is less sensitive than by IF (see Table 4.7), but allows better visualization of the tissue, which is very helpful in cases in which C4d studies by IF are equivocal, such as in biopsies with extensive scarring or necrosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


