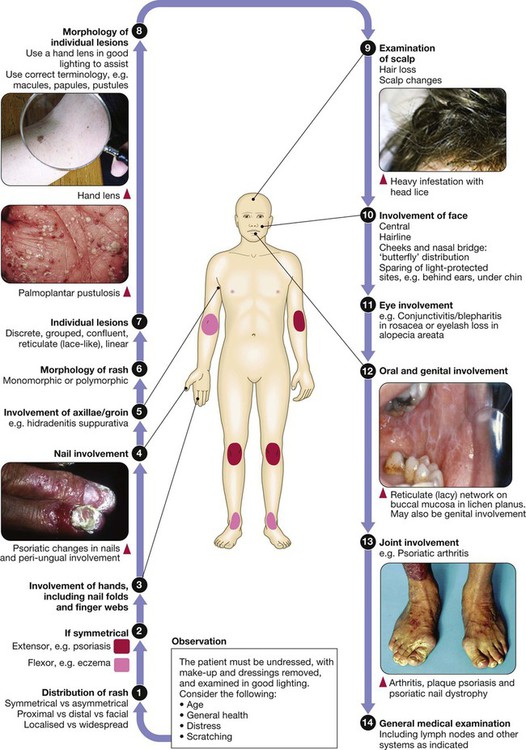
Disease affecting the human skin is common, and is important because the absence of normal skin function, as well as sometimes being life-threatening, can severely impair quality of life. This may be exacerbated by the fact that people with skin disease may suffer the effects of stigma, on occasion stemming from others’ belief that skin changes are the result of contagious disease. Skin diseases affect all ages and number more than two thousand. Assessment of the skin is valuable in the management of anyone presenting with any medical problem and, conversely, assessment of the other body systems is important when managing primarily skin disease. This chapter concentrates on those skin diseases seen most frequently and those that are important as components of general medical conditions affecting other organ systems along with the skin. Skin infections, including those related to the human immunodeficiency virus (HIV), are also discussed in Chapters 13, 14 and 15. On most sites, the epidermis is only 0.1–0.2 mm thick, except on the palms or soles where it can extend to several millimetres. Keratinocytes make up approximately 90% of epidermal cells (Fig. 28.1). The main proliferative compartment is the basal layer. Keratinocytes synthesise a range of structural proteins, such as keratins, loricrin and filaggrin (filament aggregating protein), that play key roles in maintaining normal cutaneous physiology. There are more than 50 types of keratin and their expression varies by body site, site within the epidermis and disease state. Mutations of certain keratin genes can result in blistering disorders (p. 1291) and ichthyosis (characterised by scale without major inflammation). As keratinocytes migrate from the basal layer, they differentiate, producing a variety of protein and lipid products. Keratinocytes undergo apoptosis in the granular layer before losing their nuclei and becoming the flattened corneocytes of the stratum corneum (keratin layer). The epidermis is a site of lipid production, and the ability of the stratum corneum to act as a hydrophobic barrier is the result of its ‘bricks and mortar’ design; dead corneocytes with highly cross-linked protein membranes (‘bricks’) lie within a metabolically active lipid layer synthesised by keratinocytes (‘mortar’). Terminal differentiation of keratinocytes relies on the keratin filaments being aggregated and this is, in part, mediated by filaggrin. Mutations of the filaggrin gene are found in icthyosis vulgaris, and occur in some patients with atopic eczema (p. 1283). The skin is a barrier against physical stresses. Cell-to-cell attachments must be able to transmit and dissipate stress, a function performed by desmosomes. Diseases that affect desmosomes, such as pemphigus (p. 1294), result in blistering due to keratinocyte separation. The remaining 10% of epidermal cells are: • Langerhans’ cells: dendritic, bone marrow-derived cells that circulate between the epidermis and local lymph nodes. Their prime function is antigen presentation to lymphocytes. Other dermal antigen-presenting dendritic cells are also present. • Melanocytes: predominantly in the basal layer and of neural crest origin. They synthesise the pigment melanin from tyrosine, package it in melanosomes and transfer it to surrounding keratinocytes via their dendritic processes. • Merkel cells: occur in the basal layer and are thought to play a role in signal transduction of fine touch. Their embryological derivation is unclear. The basement membrane (see Fig. 28.1) is an anchor for the epidermis and allows movement of cells and nutrients between dermis and epidermis. The cell membrane of the epidermal basal cell is attached to the basement membrane via hemi-desmosomes. The lamina lucida lies immediately below the basal cell membrane and is composed predominantly of laminin. Anchoring filaments extend through the lamina lucida to attach to the lamina densa. This electron-dense layer consists mostly of type IV collagen; from it extend loops of type VII collagen, forming anchoring fibrils that fasten the basement membrane to the dermis. Patch testing investigates delayed, cell-mediated, type IV hypersensitivity, which manifests as dermatitis. It is a provocation test with potential allergens (see Box 28.26, p. 1285) applied, at concentrations and in vehicles to minimise false positive and false negative reactions, under occlusion to the back for 48 hours before examination. When interpreting patch test readings, it is important to determine the clinical relevance of any allergic reactions before giving avoidance advice. Prick tests are used to investigate cutaneous type I (immediate) hypersensitivity to various antigens such as pollen, house dust mite or dander. Skin is pricked with commercially available stylets through a dilution of the appropriate antigen solution (p. 90). Alternatively, specific immunoglobulin E (IgE) levels to antigens can be measured in serum, and occasionally challenge tests are undertaken (p. 90). Phototesting is important for assessing suspected photosensitivity. The mainstay investigation is monochromator phototesting, which involves exposing the patient’s back to increasing doses of irradiation using narrow wavebands across the solar spectrum and then assessing responses, using the minimal erythema dose (MED) at each waveband. This is the dose required to cause just perceptible skin reddening and is compared with values for the normal population. If a patient has reduced MEDs (i.e. develops erythema at lower doses than healthy subjects), this indicates abnormal photosensitivity. Thus, monochromator phototesting can be used to determine whether a patient is abnormally photosensitive, which wavebands are involved and how sensitive the patient is (p. 1260). Provocation testing can be performed with a broadband (usually UVA) source to induce rash at a test site (most useful for polymorphic light eruption) and can be helpful for diagnosis. Patients who are referred for phototherapy will also usually undergo an MED test, in which they are exposed to a series of test doses of the light source that will be used therapeutically (often narrowband UVB), and the MED is determined 24 hours later (or 72–96 hours for the psoralen–ultraviolet A (PUVA) minimal phototoxic dose) (p. 1266). This allows treatment regimens to be individualised, based on a patient’s erythemal responses, and may detect abnormal photosensitivity. A ‘new or changed lesion’ is one of the key dermatology presentations. The challenge is to distinguish between benign and malignant disease (p. 1269). Detailed history-taking and examination are essential: • Lesion: Is this new or has a pre-existing lesion changed? What is the nature of the change – is it in size, colour, shape or surface? Has change been rapid or slow? Are there other features – pain, itch, inflammation, bleeding or ulceration? • Patient: What is the patient’s age? Are they fair-skinned and freckled? Has there been much sun exposure? Have they used sunbeds or lived in sunny climates? Have they used photoprotection? • Site: Is it sun-exposed or covered? The scalp, face, upper limbs and back in men, and face, hands and lower legs in women, are the most chronically sun-exposed sites. • Are there other similar lesions? These might include actinic keratoses (see Fig. 28.11, p. 1271) or basal cell papillomas (see Fig. 28.15, p. 1274). • Morphology of the lesion: Tenderness, size, symmetry, regularity of border, colour, surface characteristics and the presence of features such as crust, scale and ulceration must be assessed. Stretching the skin and using a magnifying lens can be helpful, e.g. in detecting the raised, pearled edge of a basal cell carcinoma (p. 1270). • Dermatoscopy: This can be used to detect the presence of abnormal vessels, such as in basal cell carcinoma or the characteristic keratin cysts in basal cell papillomas. It is invaluable for assessing pigmented and vascular lesions (Fig. 28.2). This is a common differential and one that it is critical to resolve correctly. • The precise nature of the change should be determined (as above). Listen to the patient and pay attention to subtle changes, as patients know their skin well. • If the patient has other pigmented lesions, then these should be examined too, as they may be informative. For example, if the presenting lesion looks different from the others, then suspicion is needed; conversely, if the patient has multiple basal cell papillomas, this may be reassuring – although do not be falsely reassured. • Is there a positive family history of melanoma? A suspicious naevus in a patient with a first-degree relative with melanoma probably warrants excision. The ABCDE ‘rule’ is a guide to the characteristic features of melanoma (Box 28.3 and see Fig. 28.2), although, ideally, melanomas should be diagnosed before the diameter is greater than 0.5 cm. Loss of normal skin markings in a pigmented lesion may be suggestive of melanoma. Conversely, normal skin markings and fine hairs dispersed evenly over a lesion are reassuring but do not exclude melanoma. The Glasgow seven-point checklist is another useful guide: A rash is the other common presentation in dermatology. The main categories of scaly rashes are listed in Box 28.4. Diagnosis can often be made on clinical grounds, although a biopsy may be required. Important aspects of the history are: • Age at onset and duration of rash? For example, atopic eczema often starts in early childhood and psoriasis between 15 and 40 years, and both may be chronic. Infective or drug-induced rashes are more likely to be of short duration and the latter occur in relation to drug ingestion. Duration of individual lesions is also important, as, for example, in urticaria. • Body site at onset and distribution? For example, flexural sites are involved in atopic eczema and extensor surfaces and scalp in psoriasis. Symmetry is often indicative of an endogenous disease, such as psoriasis, whereas asymmetry is more common with exogenous causes, such as contact dermatitis or infections like herpes zoster. • Is it itchy? For example, eczema is usually extremely itchy and psoriasis less so. • Was there a preceding illness or were systemic symptoms present? Examples include guttate psoriasis precipitated by a β-haemolytic streptococcal throat infection; almost all patients with infectious mononucleosis (p. 320) treated with amoxicillin will develop an erythematous maculopapular eruption; the rash of secondary syphilis follows a history of chancre at the site of inoculation; malaise and arthralgia are common in drug eruptions and vasculitis. The morphology of the rash and the characteristics of individual lesions are important (see Box 28.4). There are a limited number of conditions that present with blisters (Box 28.5). Blistering occurs due to loss of cell adhesion within the epidermis or sub-epidermal region (see Fig. 28.1, p. 1253), and the clinical presentation depends on the site or level of blistering within the skin, which in turn reflects the underlying pathogenesis (p. 1291): • Intact blisters are not often seen if the split is high in the epidermis (below the stratum corneum), as the blister roof is so fragile that it ruptures easily, leaving erosions (e.g. pemphigus foliaceus, staphylococcal scalded skin syndrome (see Fig. 28.18, p. 1276) and bullous impetigo). • If the split is lower in the epidermis, then intact flaccid blisters and erosions may be seen (e.g. pemphigus vulgaris and toxic epidermal necrolysis (see Fig. 28.36, p. 1292)). • If the split is sub-epidermal, then tense-roofed blisters occur (e.g. bullous pemphigoid (see Fig. 28.37, p. 1294), epidermolysis bullosa acquisita and porphyria cutanea tarda (see Fig. 28.47, p. 1302)). • If there are foci of separation at different levels of the epidermis, as in a dermatitis (p. 1283), multilocular bullae (made up of coalescing vesicles) occur. A systematic approach to diagnosis is required: 1. Exclude infection: e.g. herpes simplex, varicella zoster or Staph. aureus. 2. Consider common skin disorders in which blistering uncommonly occurs: e.g. severe peripheral oedema, cellulitis, allergic contact dermatitis and eczema. 3. Remember that bullae may develop in drug eruptions: e.g. fixed drug eruption (p. 1303), erythema multiforme (p. 1302) and vasculitis (p. 1115). Toxic epidermal necrolysis (TEN) is a medical emergency (p. 1292). 4. Consider immunobullous disease (p. 1292): the age of the patient may be informative (see Box 28.35, p. 1293). Diagnosis is important and full assessment, through history, examination and, sometimes, investigations, is necessary. When a patient presents with generalised itch, it is important to determine whether skin changes are primary (a process in the skin causing itch) or secondary (skin changes caused by rubbing and scratching because of itch). Many common primary skin disorders are associated with itch (Box 28.6). If itch is not connected with primary skin disease, many causes should be considered (Box 28.7); these include liver diseases (mainly cholestatic diseases, such as primary biliary cirrhosis), malignancies (e.g. generalised itch may be the presenting feature of lymphoma), haematological conditions (e.g. generalised itch in chronic iron deficiency or water contact-provoked (aquagenic) intense itch in polycythaemia), endocrine diseases (including hypo- and hyperthyroidism), chronic kidney disease (in which severity of itch is not always clearly associated with plasma creatinine concentration) and psychogenic causes (such as in ‘delusions of infestation’). Pruritus is common in pregnancy and may be due to one of the pregnancy-specific dermatoses. Diagnosis is particularly important in pregnancy, as some disorders can be associated with increased fetal risk (Box 28.8). Cutaneous photosensitivity is an abnormal response of the skin to ultraviolet (UVR) or visible radiation. The sun is the natural source but patients may also be exposed to artificial sources of UVR through the use of sunbeds and/or phototherapy (p. 1266). Chronic UVR exposure increases skin cancer risk and photoageing (p. 1254). Acute exposure can induce erythema (redness) as a normal response (Fig. 28.3). However, abnormal photosensitivity occurs when a patient reacts to lower doses than would normally cause a response, either with a heightened erythemal reaction or the development of a rash. Photoaggravated skin diseases are exacerbated by sunlight but not caused by it. The main photosensitive and photoaggravated diseases are listed in Box 28.9.
Skin disease
Clinical examination in skin disease
Functional anatomy and physiology
Epidermis
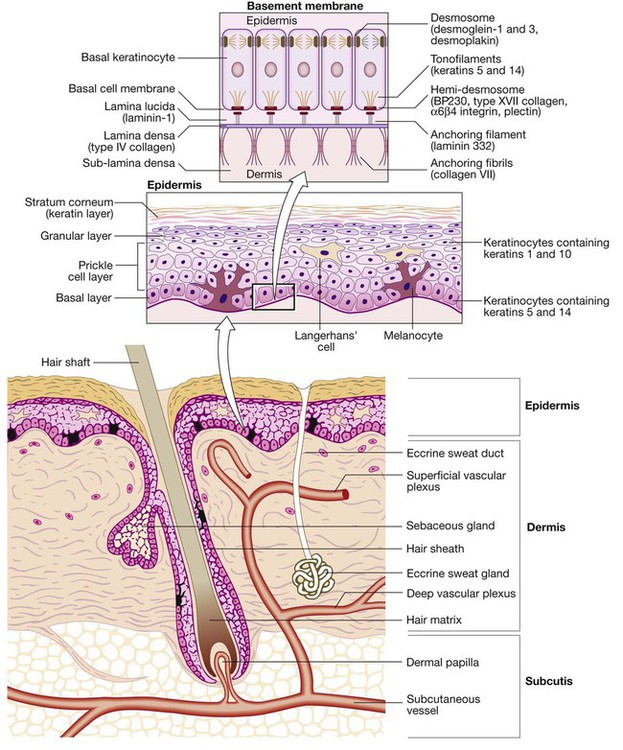
Basement membrane
Investigations
Patch testing
Prick tests and specific immunoglobulin E testing
Phototesting
Presenting problems in skin disease
Lumps – new or changing lesions
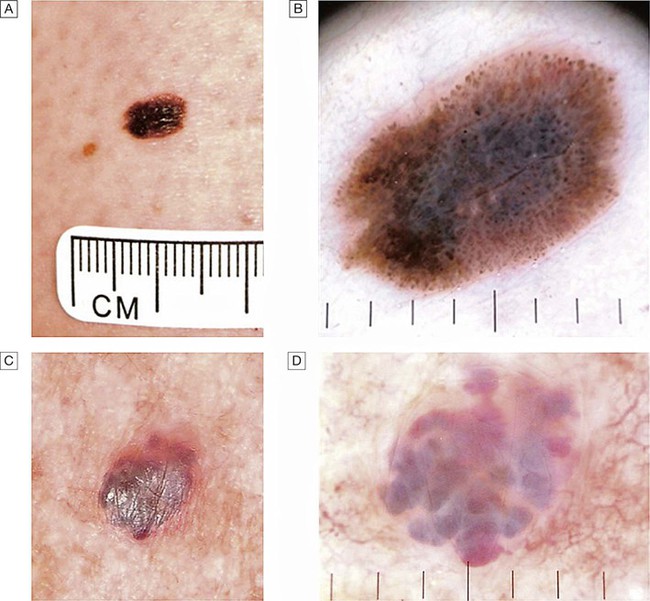
A A changing lesion. B Dermatoscopy highlights the abnormal pigment network and other features suggestive of melanoma. Excision biopsy confirmed the diagnosis of superficial spreading malignant melanoma (Breslow thickness 0.8 mm). C Another changing lesion. D Dermatoscopy highlights the vascular lacunae of this benign angioma and the patient was reassured.
Is it a melanocytic naevus or a malignant melanoma?
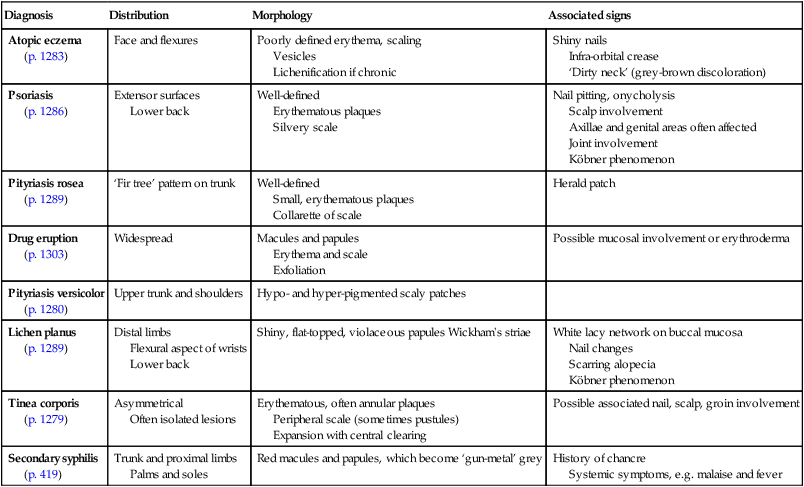
Rashes – papulosquamous eruptions
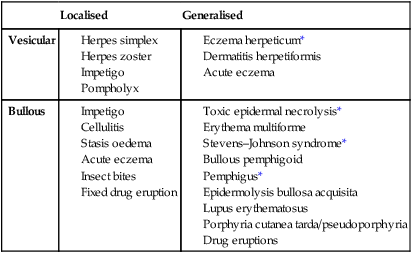
Blisters
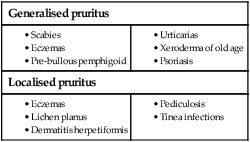
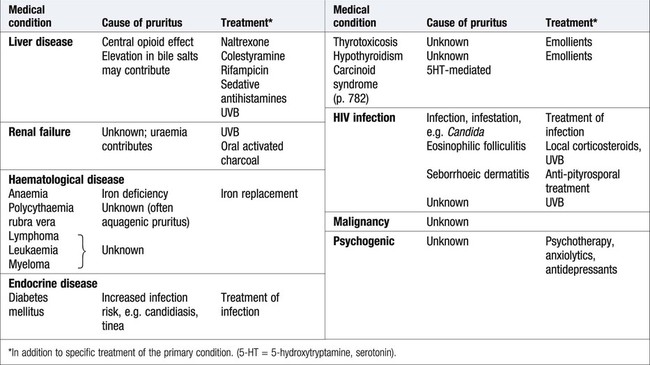
Itch (pruritus)
Clinical assessment
Photosensitivity