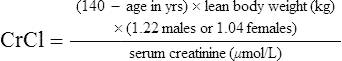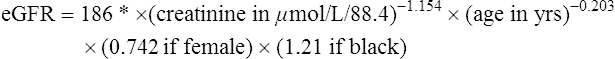17 This chapter describes the disorders of the kidneys and urinary tract that are commonly encountered in routine practice, as well as giving an overview of the highly specialised field of renal replacement therapy. Disorders of renal tubular function, which may cause alterations in electrolyte and acid–base balance, are described in Chapter 16. Each kidney is approximately 11–14 cm in length in healthy adults; they are located retroperitoneally on either side of the aorta and inferior vena cava between the 12th thoracic and 3rd lumbar vertebra (Fig. 17.1A). The right kidney is usually a few centimetres lower because the liver lies above it. Both kidneys rise and descend several centimetres with respiration. The kidneys have a rich blood supply and receive approximately 20–25% of cardiac output through the renal arteries, which arise from the abdominal aorta. The renal arteries undergo various subdivisions within the kidney, eventually forming interlobular arteries that run through the renal cortex. These eventually give rise to afferent glomerular arterioles that supply individual nephrons, which are the functional units of the kidney. The efferent arteriole, leading from the glomerulus, supplies the distal nephron and medulla in a ‘portal’ circulation (Fig. 17.1B). Healthy kidneys contain approximately 1 million individual nephrons. Each nephron consists of a glomerulus, which is responsible for ultrafiltration of blood, a proximal renal tubule, a loop of Henle, a distal renal tubule and a collecting duct, which together are responsible for selective reabsorption of water and electrolytes that have been filtered at the glomerulus (see Fig. 16.2, p. 430). Under normal circumstances, more than 99% of the 170 litres of glomerular filtrate that is produced each day is reabsorbed in the tubules. The remainder passes through the collecting ducts of multiple nephrons and drains into the renal pelvis and ureters. The glomerulus comprises a tightly packed loop of capillaries supplied by an afferent arteriole and drained by an efferent arteriole. It is surrounded by a cup-shaped extension of the proximal tubule termed Bowman’s capsule, which is comprised of epithelial cells. Blood that enters the glomerulus undergoes ultrafiltration across the glomerular basement membrane (GBM), which is formed by fusion of the basement membranes of tubular epithelial and vascular endothelial cells (Fig. 17.1C and D). The glomerular capillary endothelial cells contain pores (fenestrae), through which circulating molecules can pass to reach the underlying GBM. Glomerular epithelial cells (podocytes) have multiple long foot processes which interdigitate with those of the adjacent epithelial cells (Fig. 17.1E). As well as maintaining a selective barrier to filtration, podocytes are involved in regulating turnover of the GBM. Mesangial cells lie in the central region of the glomerulus. They have contractile properties similar to those of vascular smooth muscle cells but also have macrophage-like properties. Filtration pressure at the glomerulus is normally maintained at a constant level, in the face of wide variations in systemic blood pressure and cardiac output, by alterations in muscle tone within the afferent and efferent arterioles. This is known as autoregulation. When there is a reduction in renal perfusion pressure, renin is released by specialised smooth muscle cells in the juxtaglomerular apparatus. Renin cleaves angiotensinogen to release angiotensin I, which is further cleaved by angiotensin-converting enzyme (ACE) to produce angiotensin II (Fig. 17.1D). This restores glomerular perfusion pressure in the short term by causing vasoconstriction of the efferent arterioles within the kidney and by inducing systemic vasoconstriction to increase blood pressure and thus renal perfusion pressure. In the longer term, angiotensin II increases plasma volume by stimulating aldosterone release, which enhances sodium reabsorption by the renal tubules (see Fig. 20.17, p. 771). The proximal renal tubule, loop of Henle, distal renal tubule and collecting ducts are responsible for reabsorption of water, electrolytes and other solutes, as well as regulating acid–base balance, as described in detail on page 430 and in Figure 16.3. They also play a key role in regulating calcium homeostasis by converting 25-hydroxyvitamin D to the active metabolite 1,25-dihydroxyvitamin D (p. 1126). Failure of this process contributes to the pathogenesis of hypocalcaemia and bone disease which occurs in chronic kidney disease (CKD, p. 483). Fibroblast-like cells that lie in the interstitium of the renal cortex are responsible for production of erythropoietin, which in turn is required for production of red blood cells. Erythropoietin synthesis is regulated by oxygen tension; anaemia and hypoxia increase production, whereas polycythaemia and hyperoxia inhibit it. Failure of erythropoietin production plays an important role in the pathogenesis of anaemia in CKD. The ureters drain urine from the renal pelvis (Fig. 17.1A) and deliver it to the bladder, a muscular organ that lies anteriorly in the lower part of the pelvis, just behind the pubic bone. The function of the bladder is to store and then release urine during micturition. The bladder is richly innervated. Sympathetic nerves arising from T10–L2 relay in the pelvic ganglia to cause relaxation of the detrusor muscle and contraction of the bladder neck (both via α-adrenoceptors), thereby preventing release of urine from the bladder. The distal sphincter mechanism is innervated by somatic motor fibres from sacral segments S2–4, which reach the sphincter either by the pelvic plexus or via the pudendal nerves. Afferent sensory impulses pass to the cerebral cortex, from where reflex-increased sphincter tone and suppression of detrusor contraction inhibit micturition until it is appropriate. Conversely, parasympathetic nerves arising from S2–4 stimulate detrusor contraction, promoting micturition. The glomerular filtration rate (GFR) is the rate at which fluid passes into nephrons after filtration and is a measure of renal function. It is proportionate to body size and the reference range is usually expressed after correction for body surface area as 120 ± 25 mL/min/1.73 m2. The GFR may be measured directly by injecting and measuring the clearance of compounds such as inulin or radiolabelled ethylenediamine-tetracetic acid, which are completely filtered at the glomerulus and are not secreted or reabsorbed by the renal tubules (Box 17.1). However, this is not performed routinely and is usually reserved for special circumstances, such as the assessment of renal function in potential live kidney donors. Instead, GFR is usually indirectly assessed in clinical practice by measuring serum levels of endogenously produced compounds that are excreted by the kidney. The most widely used is serum creatinine, which is produced by muscle at a constant rate, is almost completely filtered at the glomerulus, and is not reabsorbed. Although creatinine is secreted to a small degree by the proximal tubule, this is only usually significant in terms of GFR estimation in severe renal impairment, where it accounts for a larger proportion of the creatinine excreted. Accordingly, provided muscle mass remains constant, changes in serum creatinine concentrations closely reflect changes in GFR, although the reference range for creatinine is wide due to the fact that muscle mass varies widely between different individuals (Fig. 17.2). Several methods have been developed with which to estimate GFR from serum creatinine measurements (see Box. 17.1) but the most widely used is the MDRD equation, which is now the accepted standard for assessing estimated GFR (eGFR). Although the eGFR has several limitations (Box 17.2), its routine reporting by laboratories has increased recognition of moderate kidney damage and encouraged early deployment of protective therapies (Box 17.3). A potentially more accurate assessment of GFR can be obtained by collection of a 24-hour urine sample and relating serum creatinine levels to urinary creatinine excretion (see Box 17.1). Screening for the presence of blood, protein, glucose, ketones, nitrates and leucocytes and to assess pH and osmolality of urine can be achieved by dipstick testing (p. 474). Urine microscopy (p. 463) or flow cytometry can detect erythrocytes, which are indicative of bleeding from the urogenital tract (anywhere from kidney to tip of penis); dysmorphic erythrocytes, which suggest the presence of nephritis; red cell casts, indicative of glomerular disease; and crystals, which may be observed in patients with renal stone disease. It should be noted that calcium oxalate and urate crystals can sometimes be found in normal urine that has been left to stand, due to crystal formation ex vivo. The presence of leucocytes and bacteria in urine is indicative of renal tract infection. White cell casts are strongly suggestive of pyelonephritis. Urine pH can provide diagnostic information in the assessment of renal tubular acidosis (p. 446). Urine collection over a 24-hour period can be performed to measure excretion of solutes, such as calcium, oxalate and urate, in patients with recurrent renal stone disease (p. 507). Proteinuria can also be measured on 24-hour collections but is usually now quantified by protein/creatinine ratio on spot urine samples. Other dynamic tests of tubular function, including concentrating ability (p. 794), ability to excrete a water load (p. 438) and ability to excrete acid (p. 426), and calculation of fractional calcium, phosphate or sodium excretion, are valuable in some circumstances. The fractional excretion of these ions can be calculated by the general formula: (urine concentration of analyte × serum creatinine) / (serum concentration of analyte × urinary creatinine). For example, familial benign hypercalcaemia is characterised by a very low fractional excretion of calcium (p. 770), and hypophosphataemic rickets (p. 1128) by an increased fractional excretion of phosphate. Calculation of fractional excretion of sodium (FENa) can help differentiate volume depletion, when the tubules are avidly conserving sodium (FENa typically < 1.0), from acute tubular necrosis, when the tubules are damaged and are less able to conserve sodium (FENa typically > 1.0). Abnormalities of routine biochemistry are common in renal disease. Serum levels of creatinine may be raised, reflecting reduced GFR (see above), although serum creatinine values can remain within the reference range in patients with reduced muscle mass, even when the GFR has fallen by more than 50%. Serum levels of urea are often increased in kidney disease but this analyte has limited value as a measure of GFR since levels increase with protein intake, following gastrointestinal haemorrhage and in catabolic states. Conversely, urea levels may be reduced in patients with liver failure or anorexia and in malnourished patients, independently of changes in renal function. Serum calcium tends to be reduced and phosphate increased in CKD, in association with high parathyroid hormone (PTH) levels caused by reduced production of 1,25(OH)2D by the kidney (secondary hyperparathyroidism). In some patients, this may be accompanied by raised serum alkaline phosphatase levels, which are indicative of renal osteodystrophy. Other biochemical abnormalities may be observed that reflect underlying disease processes, such as raised glucose and HbA1c levels in diabetes mellitus (p. 807) and raised levels of C-reactive protein (CRP) in sepsis and vasculitis. Antinuclear antibodies, antibodies to extractable nuclear antigens and anti-double-stranded DNA antibodies may be detected in patients with renal disease secondary to SLE (p. 1109). Antineutrophil cytoplasmic antibodies (ANCA) may be detected in patients with glomerulonephritis secondary to systemic vasculitis (p. 1115), as may antibodies to GBM in patients with Goodpasture’s syndrome (p. 497) and low levels of complement in SLE, systemic vasculitis and HUS. Renal ultrasound is a valuable non-invasive technique that is indicated to assess renal size and to investigate patients who are suspected of having obstruction of the urinary tract (Fig. 17.3) or renal tumours, cysts or stones. It is often the only method required for renal imaging and has the advantage of showing other abdominal, pelvic and retroperitoneal pathology. Ultrasound can also be used to provide images of the prostate gland and bladder, and to estimate the completeness of emptying in patients with suspected bladder outflow obstruction. Ultrasonography may show increased density of the renal cortex with loss of distinction between cortex and medulla, which is characteristic of CKD. Doppler imaging can be used to study blood flow in extrarenal and larger intrarenal vessels and can assess the resistivity index, which is the ratio of peak systolic and diastolic velocity. This is influenced by the resistance to flow through small intrarenal arteries and may be elevated in various diseases, including acute glomerulonephritis and rejection of a renal transplant. High peak velocities can also occur in severe renal artery stenosis. However, renal ultrasound is operator-dependent, the stored images convey only a fraction of the dynamic information gained during the investigation, and the results are often less clear in obese patients. Computed tomography urography (CTU) is used to evaluate cysts and mass lesions in the kidney or filling defects within the collecting systems. It usually entails an initial scan without contrast medium, and subsequent scans following injection of contrast to obtain a nephrogram image and images during the excretory phases. This technique gives more information than intravenous urography (IVU) but entails a substantially larger radiation dose. Contrast enhancement is particularly useful for characterising mass lesions within the kidney and differentiating benign from malignant lesions (see Fig. 17.32A, p. 516). Computed tomography without contrast (CT) gives clear definition of retroperitoneal anatomy regardless of obesity and is superior to ultrasound in this respect. Non-contrast CT of kidneys, ureters and bladder (CTKUB) is the method of choice for demonstrating stones within the kidney or ureter. This technique (CT-angiography) involves performing computed tomography, following an intravenous injection of contrast medium, to obtain images of the renal vasculature. It produces high-quality images of the main renal vessels and is of value in patients who have suffered renal trauma and those with haemorrhage from the renal tract, and in the investigation of renal artery stenosis. Other vascular structures, such as angiomyolipomas and aneurysms, can also be detected. Drawbacks include the fact that relatively large doses of contrast medium are required, which can cause renal dysfunction, and that the radiation dose is significant (Box 17.4). Magnetic resonance imaging (MRI) offers excellent resolution and gives good distinction between different tissue types (see Fig. 17.26, p. 506). It is very useful for local staging of prostate, bladder and penile cancers. Magnetic resonance angiography (MRA) provides an alternative to CT-angiography for imaging renal vessels but involves administration of gadolinium-based contrast media, which may carry risks for patients with impaired renal function (see Box 17.4). Whilst MRA gives good images of the main renal vessels, stenosis of small branch arteries may be missed. Renal arteriography involves taking X-rays following an injection of contrast medium directly into the renal artery. The main indication is to investigate renal artery stenosis (p. 494) or haemorrhage. Renal angiography can often be combined with therapeutic balloon dilatation or stenting of the renal artery and can be used to occlude bleeding vessels and arteriovenous fistulae by the insertion of thin platinum wires (coils). These curl up within the vessel and promote thrombosis, thereby securing haemostasis. Intravenous urography (IVU) involves taking serial plain X-rays immediately before and after an intravenous injection of contrast medium. It has largely been replaced by ultrasound, CTKUB and CTU for most renal imaging purposes but remains a useful method of viewing the renal papillae, stones and urothelial malignancies (Fig. 17.4). The initial X-rays may show the renal outlines (if perinephric fat and bowel gas shadows allow), as well as radio-opaque calculi and calcification within the renal tract. Early films taken 1 minute after injection can be used to assess renal perfusion, whereas films at later time points provide images of the collecting system, ureters and bladder. The disadvantages of this technique are the need for an injection, dependence on adequate renal function, and exposure to irradiation and contrast medium (see Box 17.4). Pyelography involves direct injection of contrast medium into the collecting system from above or below. It offers the best views of the collecting system and upper tract, and is sometimes used to identify the cause of urinary tract obstruction (p. 472). Antegrade pyelography requires the insertion of a fine needle into the pelvicalyceal system under ultrasound or radiographic control. This approach is much more difficult and hazardous in a non-obstructed kidney. In the presence of obstruction, percutaneous nephrostomy drainage can be established, and often stents can be passed through any obstruction. Retrograde pyelography can be performed by inserting catheters into the ureteric orifices at cystoscopy (Fig. 17.5). Static radionucleotide studies are performed with dimercaptosuccinic acid labelled with technetium (99mTc-DMSA), which is taken up by proximal tubular cells. Following intravenous injection, images of the renal cortex are obtained that show the shape, size and relative function of each kidney (Fig. 17.6). This is a sensitive method for demonstrating cortical scarring in reflux nephropathy and a way of assessing the individual function of each kidney. Radionuclide bone scanning following the injection of methylene diphosphonate (99mTc-MDP) is indicated to assess the presence and extent of bone metastases in men with advanced prostate cancer (p. 517). Renal biopsy is used to establish the nature and extent of renal disease in order to judge the prognosis and need for treatment (Box 17.5). The procedure is performed transcutaneously with ultrasound or contrast radiography guidance to ensure accurate needle placement into a renal pole. Light microscopy, electron microscopy and immunohistological assessment of the specimen may all be required. Dysuria refers to painful urination, often described as burning, scalding or stinging, and commonly accompanied by suprapubic pain. It is often associated with frequency of micturition and a feeling of incomplete emptying of the bladder. By far the most common cause is urinary tract infection, as described on page 511. Other diagnoses that need to be considered in patients with dysuria include sexually transmitted infections (p. 411) and bladder stones (p. 507). Loin pain is often caused by musculoskeletal disease but can be a manifestation of renal tract disease; in the latter case, it may arise from renal stones, ureteric stones, renal tumours, acute pyelonephritis and urinary tract obstruction. Acute loin pain radiating anteriorly and often to the groin is termed renal colic. When combined with haematuria, this is typical of ureteric obstruction due to calculi (p. 507). When loin pain is precipitated by a large fluid intake (Dietl’s crisis), upper urinary tract obstruction caused by a congenital abnormality of the pelvi-ureteric junction (PUJ, p. 510) is often responsible. The presence of bladder enlargement in a middle-aged or elderly man suggests benign or malignant enlargement of the prostate gland as a potential cause of oliguria or anuria (pp. 515 and 517). It is important to note that about 50% of cases of acute urinary retention are seen after general anaesthesia, particularly in patients with pre-existing prostatic enlargement. Partial obstruction can be associated with a normal or even high urine volume due to chronic tubular injury, which causes loss of tubular concentrating ability. This chronic tubular injury can also produce a type 1 renal tubular acidosis (p. 446). Over time, even partial obstruction can cause tubular atrophy and irreversible renal failure. All patients with oliguria or anuria should have routine biochemistry and haematology checked. Catheterisation should be performed so that urine volumes can be accurately monitored and the urine analysed for evidence of proteinuria and red cell casts, which may be found in patients with glomerulonephritis and systemic diseases such as vasculitis or SLE. Isolated haematuria may be indicative of an obstructive uropathy secondary to urinary calculi (p. 507) or tumours affecting the renal tract. If oliguria persists without any other clear explanation, ultrasound examination should be undertaken promptly to look for evidence of obstruction. Rarely, however, the urinary tract may not be particularly dilated in patients with acute kidney injury due to obstruction because of lack of urine production. Polyuria is defined as a urine volume in excess of 3 L/day. Various underlying conditions, both renal and extrarenal, may be responsible, as outlined in Box 17.6. Investigation of polyuria includes measurement of urea, creatinine and electrolytes, glucose, calcium and albumin. A 24-hour urine collection may be helpful to confirm the severity of polyuria and the presence of nocturnal polyuria. Investigation and management of suspected diabetes insipidus are described on page 794. Nocturia is defined as waking up at night to void urine. It may be a consequence of polyuria but may also result from increased fluid intake or diuretic use in the late evening. Nocturia also occurs in CKD, and in prostatic enlargement when it is associated with poor stream, hesitancy, incomplete bladder emptying, terminal dribbling and urinary frequency due to partial urethral obstruction (p. 515). Nocturia may also occur in sleep disturbance without functional abnormalities of the urinary tract. Urinary incontinence is defined as any involuntary leakage of urine. It may occur in patients with a normal urinary tract, as the result of dementia or poor mobility, or transiently during an acute illness or hospitalisation, especially in older people (Box 17.7). Diuretics, alcohol and caffeine may worsen incontinence.
Kidney and urinary tract disease
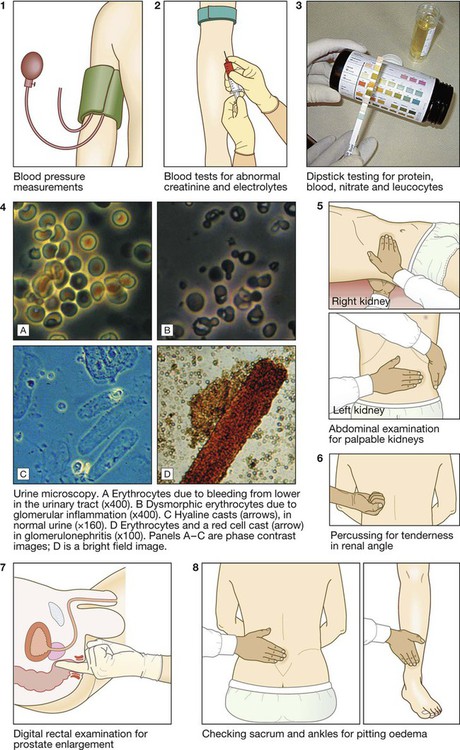
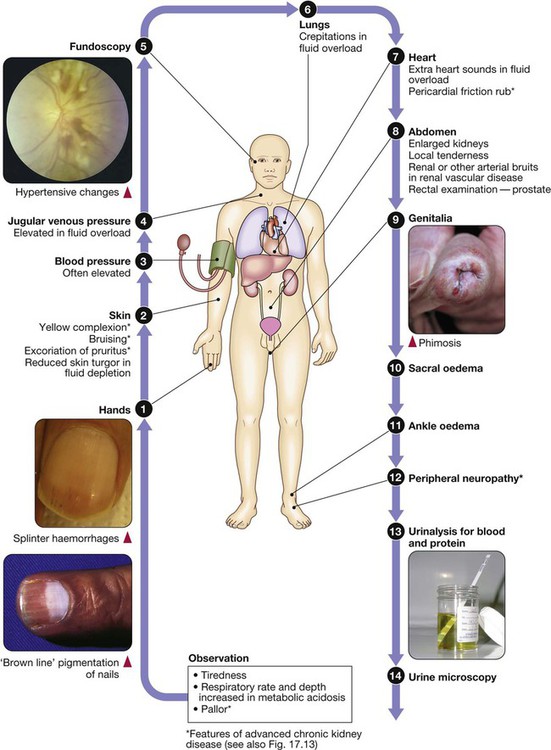
Clinical examination of the kidney and urinary tract
Functional anatomy and physiology
The kidneys
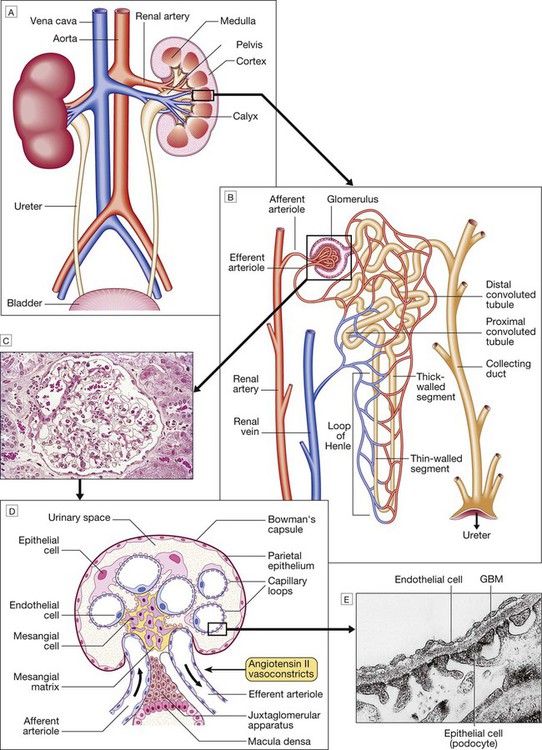
A Anatomical relationships of the kidney. B A single nephron. For the functions of different segments, see Figures 16.2 and 16.3 (pp. 430 and 431). C Histology of a normal glomerulus. D Schematic cross-section of a glomerulus, showing five capillary loops, to illustrate structure and show cell types. E Electron micrograph of the filtration barrier. (GBM = glomerular basement membrane)
The nephron
The glomerulus
Renal tubules, loop of Henle and collecting ducts
Ureters and bladder
Investigation of renal and urinary tract disease
Glomerular filtration rate
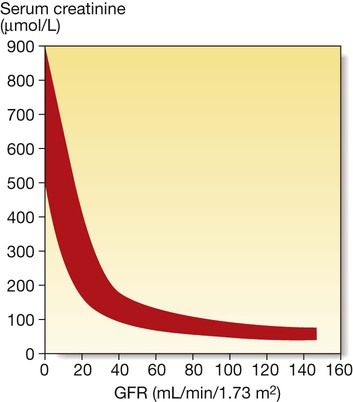
The inverse reciprocal relationship between GFR and serum creatinine is shown for a group of patients with renal disease. The red band indicates the range of values obtained. Note that some individuals have a GFR as low as 30–40 mL/min without serum creatinine rising out of the reference range.
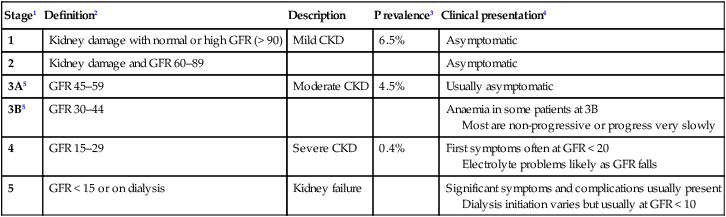
Urinalysis
Blood tests
Haematology
Biochemistry
Immunology
Imaging
Ultrasound
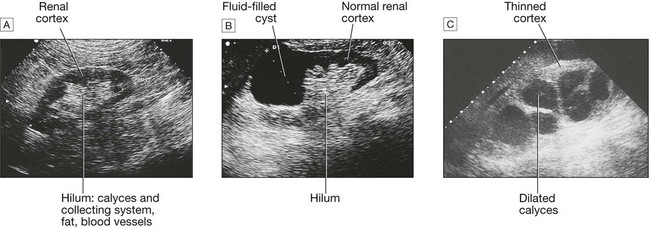
A Normal kidney. The normal cortex is less echo-dense (blacker) than the adjacent liver. B A simple cyst occupies the upper pole of an otherwise normal kidney. C The renal pelvis and calyces are dilated by a chronic obstruction to urinary outflow. The thinness and increased density of the remaining renal cortex indicate chronic changes.
Computed tomography
Computed tomography and angiography
Magnetic resonance imaging
Renal arteriography
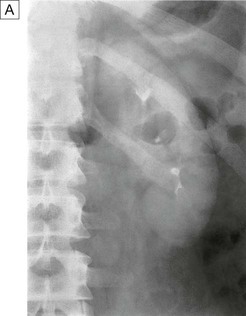
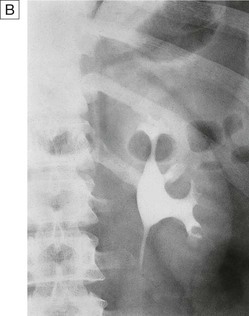
Intravenous urography
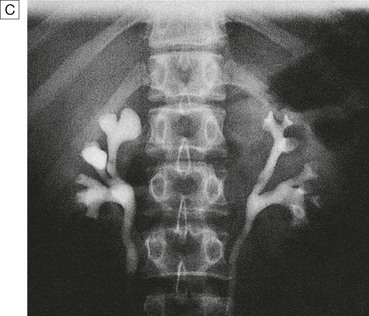
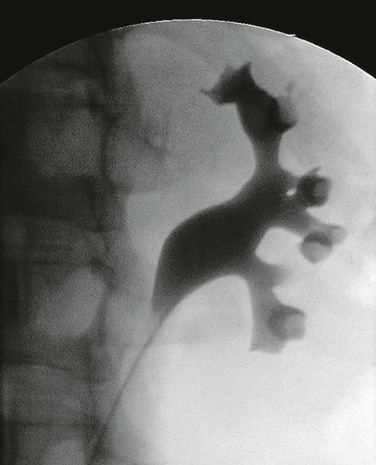
Pyelography
Radionuclide studies
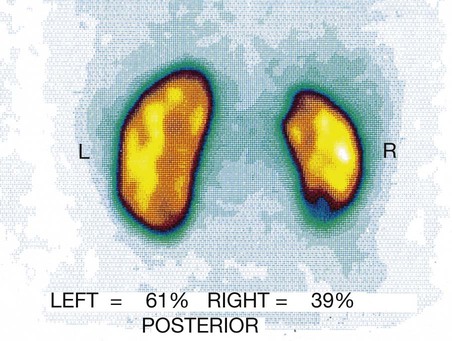
A posterior view is shown of a normal left kidney and a small right kidney (with evidence of cortical scarring at upper and lower poles) that contributes only 39% of total renal function.
Renal biopsy
Presenting problems in renal and urinary tract disease
Dysuria
Loin pain
Oliguria/anuria
Polyuria
Nocturia
Urinary incontinence
Kidney and urinary tract disease