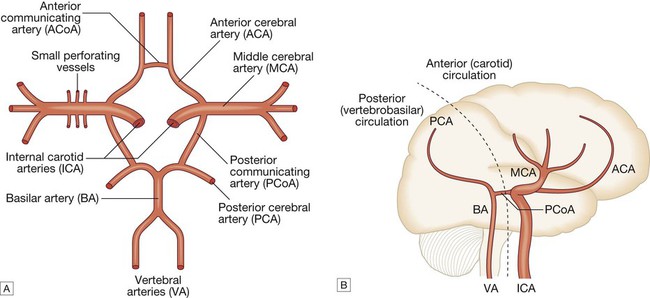
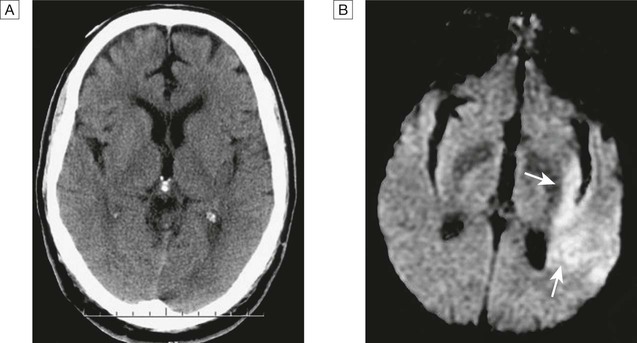
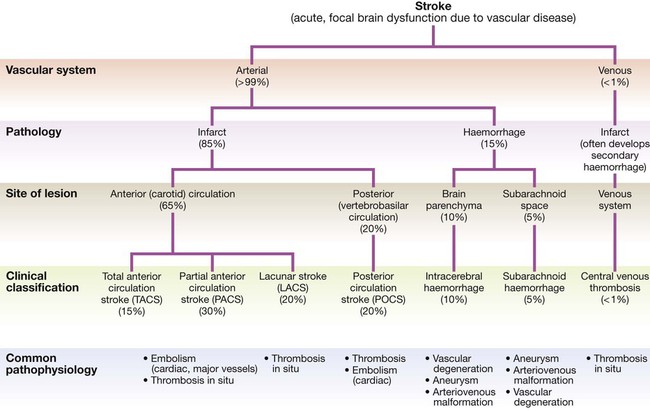
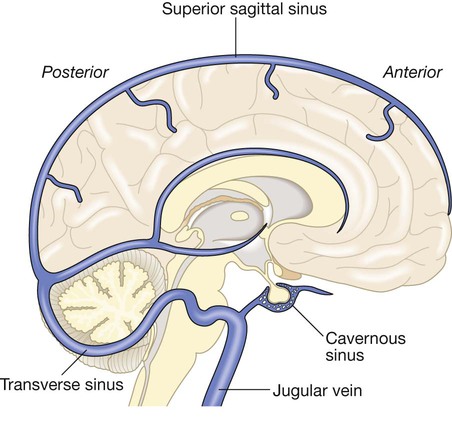
Cerebrovascular disease is the third most common cause of death in high-income countries after cancers and ischaemic heart disease, and the most common cause of severe physical disability. It includes a range of disorders of the central nervous system (Fig. 27.1). Stroke is the most common clinical manifestation of cerebrovascular disease, and results in episodes of brain dysfunction due to focal ischaemia or haemorrhage. Subarachnoid haemorrhage (SAH) and cerebral venous thrombosis (CVT) will be discussed separately, since their pathophysiology, clinical manifestations and management are distinct from those of stroke. Vascular dementia is described in Chapters 10 and 26. The main arterial supply of the brain comes from the internal carotid arteries, which supply the anterior brain, and the vertebral and basilar arteries (vertebrobasilar system), which provide the posterior circulation. The anterior and middle cerebral arteries supply the frontal and parietal lobes, while the posterior cerebral artery supplies the occipital lobe. The vertebral and basilar arteries perfuse the brain stem, mid-brain and cerebellum (Fig. 27.2). The functions of each of these areas of the brain are described on page 1141. Communicating arteries provide connections between the anterior and posterior circulations and between left and right hemispheres, creating protective anastomotic connections that form the circle of Willis. In health, regulatory mechanisms maintain a constant cerebral blood flow across a wide range of arterial blood pressures to meet the high resting metabolic activity of brain tissue; cerebral blood vessels dilate when systemic blood pressure is lowered and constrict when it is raised. This autoregulatory mechanism can be disrupted after stroke. The venous collecting system is formed by a collection of sinuses over the surface of the brain, which drain into the jugular veins (Fig. 27.3). Computed tomography (CT) scanning is the mainstay of stroke imaging. It allows the rapid identification of intracerebral bleeding and stroke ‘mimics’ (i.e. pathologies other than stroke that have similar presentations), such as tumours. Magnetic resonance imaging (MRI) is used when there is diagnostic uncertainty or delayed presentation, and when more information on brain structure and function is required (Fig. 27.4). Contraindications to MRI include cardiac pacemakers and claustrophobia on entering the scanner. These techniques are described on page 1149. Various techniques are used to obtain images of extracranial and intracranial blood vessels (Fig. 27.5). The least invasive is ultrasound (Doppler or duplex scanning), which is used to image the carotid and the vertebral arteries in the neck. In skilled hands, reliable information can be provided about the degree of arterial stenosis and the presence of ulcerated plaques. Blood flow in the intracerebral vessels can be examined using transcranial Doppler. While the anatomical resolution is limited, it is improving and many centres no longer require formal angiography before proceeding to carotid endarterectomy (see below). Blood flow can also be detected by specialised sequences in MR angiography (MRA) but the anatomical resolution is still not comparable to that of intra-arterial angiography, which outlines blood vessels by the injection of radio-opaque contrast intravenously or intra-arterially. The X-ray images obtained can be enhanced by the use of computer-assisted digital subtraction or spiral CT. Because of the significant risk of complications, intra-arterial contrast angiography is reserved for patients in whom non-invasive methods have provided a contradictory picture or incomplete information, or in whom it is necessary to image the intracranial circulation in detail: for example, to delineate a saccular aneurysm, an arteriovenous malformation or vasculitis. These help identify underlying causes of cerebrovascular disease: for example, blood glucose (diabetes mellitus), triglycerides and cholesterol (hyperlipidaemia) or full blood count (polycythaemia) in stroke. Erythrocyte sedimentation rate (ESR) and immunological tests, such as measurement of antineutrophil cytoplasmic antibodies (ANCA) (p. 1068), may be required when vasculitis is suspected. Genetic testing for rarer inherited conditions, such as CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy), may be indicated. Unilateral weakness is the classical presentation of stroke and, much more rarely, of cerebral venous thrombosis. The weakness is sudden, progresses rapidly and follows a hemiplegic pattern (see Fig. 26.18, p. 1163). There is rarely any associated abnormal movement. Reflexes are initially reduced but then become increased with a spastic pattern of increased tone (see Box 26.20, p. 1162). Upper motor neuron weakness of the face (7th cranial nerve) is often present. Dysphasia and dysarthria are the most common presentations of disturbed speech in stroke (p. 1168). Dysphasia indicates damage to the dominant frontal or parietal lobe (see Box 26.2, p. 1142), while dysarthria is a non-localising feature reflecting weakness or incoordination of the face, pharynx, lips, tongue or palate. Visual loss in stroke can be due to unilateral optic ischaemia (called amaurosis fugax if transient), caused by disturbance of blood flow in the internal carotid artery and ophthalmic artery, which leads to monocular blindness. Ischaemic damage to the occipital cortex or post-chiasmic nerve tracts results in a contralateral hemianopia (p. 1169). Damage to the non-dominant cortex often results in contralateral visuo-spatial dysfunction, such as sensory or visual neglect and apraxia (inability to perform complex tasks despite normal motor, sensory and cerebellar function; p. 1167). This is sometimes misdiagnosed as acute confusion. Of the 180–300 patients per 100 000 population presenting annually with a stroke, 85% sustain a cerebral infarction due to inadequate blood flow to part of the brain, and most of the remainder have an intracerebral haemorrhage (see Fig. 27.1). Cerebral infarction is mostly caused by thromboembolic disease secondary to atherosclerosis in the major extracranial arteries (carotid artery and aortic arch). About 20% of infarctions are due to embolism from the heart, and a further 20% are due to thrombosis in situ caused by intrinsic disease of small perforating vessels (lenticulostriate arteries), producing so-called lacunar infarctions. The risk factors for ischaemic stroke reflect the risk factors for the underlying vascular disease (Box 27.1). About 5% are due to rare causes, including vasculitis (p. 1115), endocarditis (p. 625) and cerebral venous disease (see below). Cerebral infarction takes some hours to complete, even though the patient’s deficit may be maximal shortly after the vascular occlusion. After the occlusion of a cerebral artery, infarction may be forestalled by the opening of anastomotic channels from other arterial territories that restore perfusion to its territory. Similarly, reduction in perfusion pressure leads to compensatory homeostatic changes to maintain tissue oxygenation (Fig. 27.6). These compensatory changes can sometimes prevent occlusion of even a carotid artery from having any clinically apparent effect. However, if and when these homeostatic mechanisms fail, the process of ischaemia starts, and ultimately leads to infarction unless the vascular supply is restored. As the cerebral blood flow declines, different neuronal functions fail at various thresholds (Fig. 27.7). Once blood flow falls below the threshold for the maintenance of electrical activity, neurological deficit develops. At this level of blood flow, the neurons are still viable; if the blood flow increases again, function returns and the patient will have had a transient ischaemic attack (TIA). However, if the blood flow falls further, a level is reached at which irreversible cell death starts. Hypoxia leads to an inadequate supply of adenosine triphosphate (ATP), which leads to failure of membrane pumps, thereby allowing influx of sodium and water into the cell (cytotoxic oedema) and the release of the excitatory neurotransmitter glutamate into the extracellular fluid. Glutamate opens membrane channels, allowing the influx of calcium and more sodium into the neurons. Calcium entering the neurons activates intracellular enzymes that complete the destructive process. The release of inflammatory mediators by microglia and astrocytes causes death of all cell types in the area of maximum ischaemia. The infarction process is worsened by the anaerobic production of lactic acid (Fig. 27.8) and consequent fall in tissue pH. There have been attempts to develop neuroprotective drugs to slow down the processes leading to irreversible cell death but so far these have proved disappointing.
Stroke disease
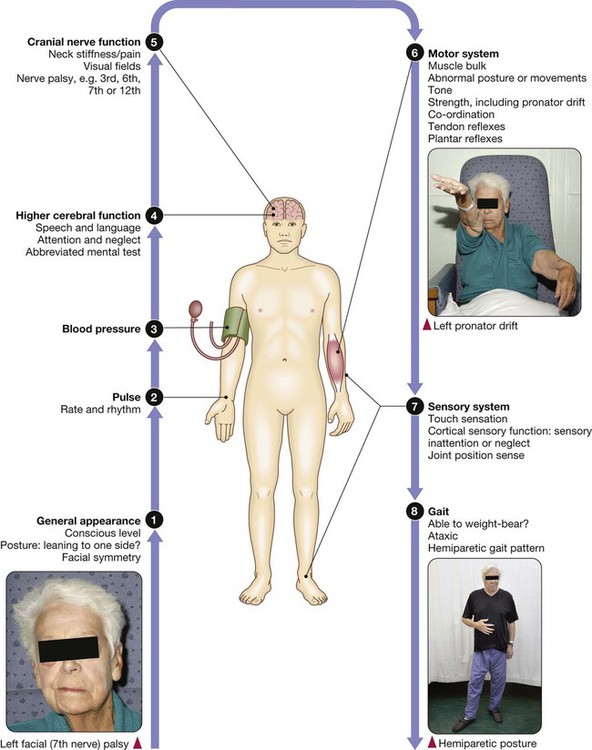
Clinical examination in stroke disease
Functional anatomy and physiology
Investigations
Neuroimaging
Vascular imaging
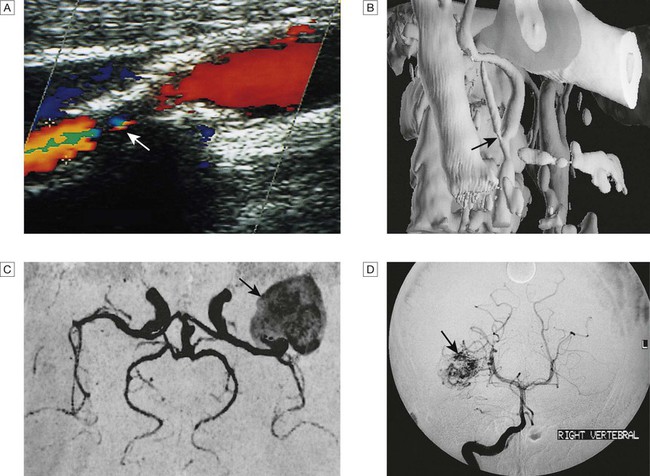
A Doppler scan showing 80% stenosis of the internal carotid artery (arrow). B Three-dimensional reconstruction of CT angiogram showing stenosis at the carotid bifurcation (arrow). C MR angiogram showing giant aneurysm at the middle cerebral artery bifurcation (arrow). D Intra-arterial angiography showing arteriovenous malformation (arrow).
Blood tests
Presenting problems
Weakness
Speech disturbance
Visual deficit
Visuo-spatial dysfunction
Stroke
Pathophysiology
Cerebral infarction
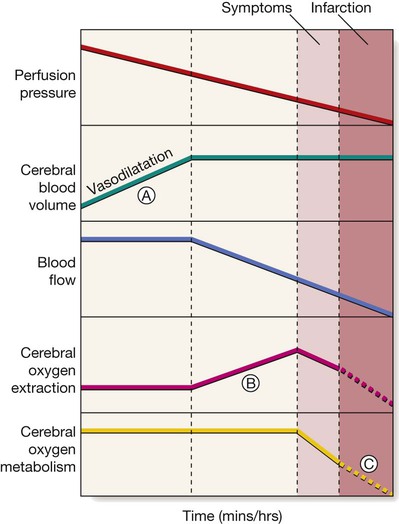
Vasodilatation initially maintains cerebral blood flow (A), but after maximal vasodilatation further falls in perfusion pressure lead to a decline in blood flow. An increase in tissue oxygen extraction, however, maintains the cerebral metabolic rate for oxygen (B). Still further falls in perfusion, and therefore blood flow, cannot be compensated; cerebral oxygen availability falls and symptoms appear, then infarction (C).
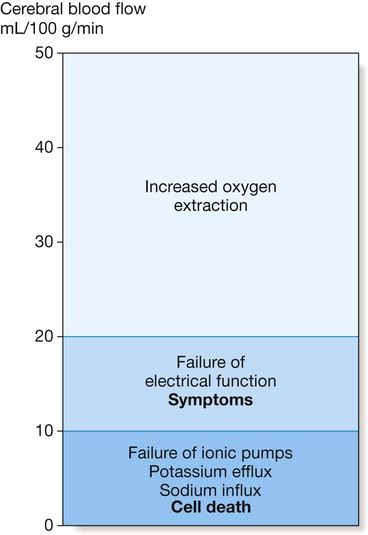
Symptoms of cerebral ischaemia appear when the blood flow has fallen to less than half of normal and energy supply is insufficient to sustain neuronal electrical function. Full recovery can occur if this level of flow is returned to normal but not if it is sustained. Further blood flow reduction below the next threshold causes failure of cell ionic pumps and starts the ischaemic cascade, leading to cell death.
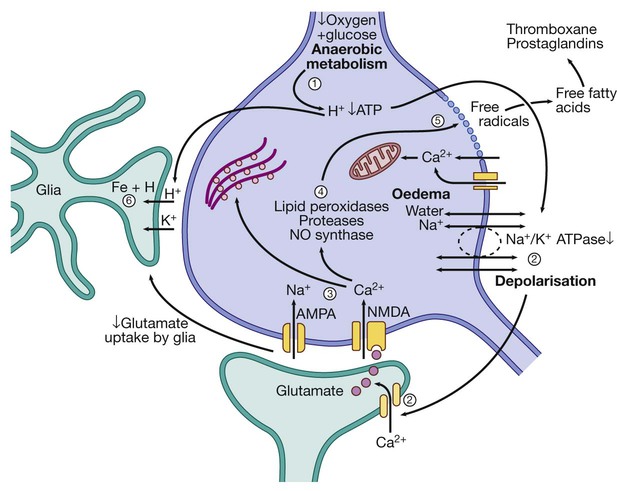
(1) Reduction of blood flow reduces supply of oxygen and hence ATP. H+ is produced by anaerobic metabolism of available glucose. (2) Energy-dependent membrane ionic pumps fail, leading to cytotoxic oedema and membrane depolarisation, allowing calcium entry and releasing glutamate. (3) Calcium enters cells via glutamate-gated channels and (4) activates destructive intracellular enzymes (5), destroying intracellular organelles and cell membrane, with release of free radicals. Free fatty acid release activates pro-coagulant pathways that exacerbate local ischaemia. (6) Glial cells take up H+, can no longer take up extracellular glutamate and also suffer cell death, leading to liquefactive necrosis of whole arterial territory.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Stroke disease