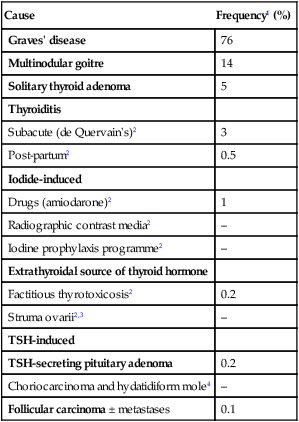
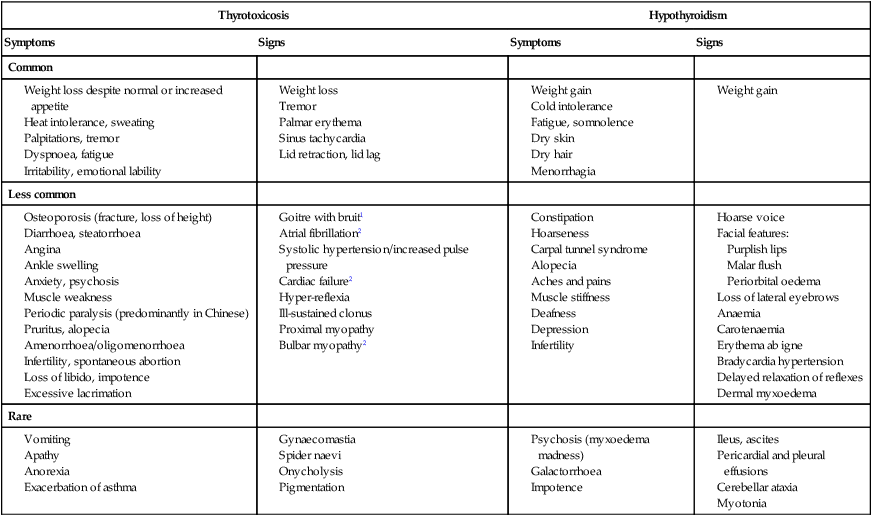
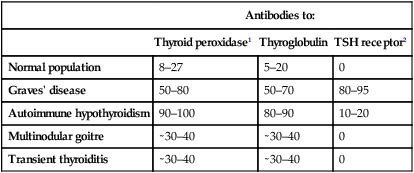
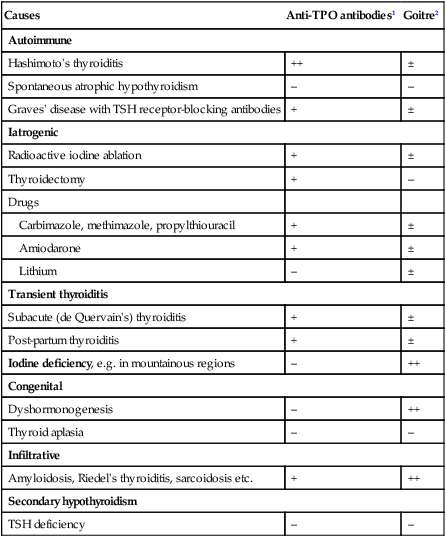
20 Diabetes mellitus (described in detail in Ch. 21) and thyroid disease are the most common endocrine disorders. Endocrinology concerns the synthesis, secretion and action of hormones. These are chemical messengers released from endocrine glands that coordinate the activities of many different cells. Endocrine diseases can therefore affect multiple organs and systems. This chapter describes the principles of endocrinology before dealing with the function and diseases of each gland in turn. Some endocrine disorders are common, particularly those of the thyroid, parathyroid glands, reproductive system and β cells of the pancreas (Ch. 21). For example, thyroid dysfunction occurs in more than 10% of the population in areas with iodine deficiency, such as the Himalayas, and 4% of women aged 20–50 years in the UK. Some endocrine diseases are becoming more common in association with emerging diseases; HIV infection is associated in particular with adrenal insufficiency. Less common endocrine syndromes are described later in the chapter. Some endocrine glands, such as the parathyroids and pancreas, respond directly to metabolic signals, but most are controlled by hormones released from the pituitary gland. Anterior pituitary hormone secretion is controlled in turn by substances produced in the hypothalamus and released into portal blood, which drains directly down the pituitary stalk (Fig. 20.1). Posterior pituitary hormones are synthesised in the hypothalamus and transported down nerve axons, to be released from the posterior pituitary. Hormone release in the hypothalamus and pituitary is regulated by numerous stimuli and through feedback control by hormones produced by the target glands (thyroid, adrenal cortex and gonads). These integrated endocrine systems are called ‘axes’, and are listed in Figure 20.2. A wide variety of molecules can act as hormones, including peptides such as insulin and growth hormone, glycoproteins such as thyroid-stimulating hormone, and amines such as noradrenaline (norepinephrine). The biological effects of hormones are mediated by binding to receptors. Many receptors are located on the cell surface. These interact with various intracellular signalling molecules on the cytosolic side of the plasma membrane to affect cell function, usually through changes in gene expression (p. 48). Some hormones, most notably steroids, triiodothyronine and vitamin D, bind to specific intracellular receptors, which directly bind to response elements on DNA to regulate gene expression (p. 42). The classical model of endocrine function involves hormones synthesised in endocrine glands, which are released into the circulation and act at sites distant from those of secretion (as in Fig. 20.1). However, additional levels of regulation are now recognised. Many other organs secrete hormones or contribute to the peripheral metabolism and activation of pro-hormones. A notable example is the production of oestrogens from adrenal androgens in adipose tissue by the enzyme aromatase. Some hormones, such as neurotransmitters, act in a paracrine fashion to affect adjacent cells, or act in an autocrine way to affect behaviour of the cell that produces the hormone. For each endocrine axis or major gland, diseases can be classified as shown in Box 20.1. Pathology arising within the gland is often called ‘primary’ disease (for example, primary hypothyroidism in Hashimoto’s thyroiditis), while abnormal stimulation of the gland is often called ‘secondary’ disease (for example, secondary hypothyroidism in patients with a pituitary tumour and thyroid-stimulating hormone deficiency). Some pathological processes can affect multiple endocrine glands (p. 795); these may have a genetic basis (such as organ-specific autoimmune endocrine disorders and the multiple endocrine neoplasia (MEN) syndromes) or be a consequence of therapy for another disease (for example, following treatment of childhood cancer with chemotherapy and/or radiotherapy). Biochemical investigations play a central role in endocrinology. Most hormones can be measured in blood, but the circumstances in which the sample is taken are often crucial, especially for hormones with pulsatile secretion, such as growth hormone; those that show diurnal variation, such as cortisol; or those that demonstrate monthly variation, such as oestrogen or progesterone. Other investigations, such as imaging and biopsy, are more frequently reserved for patients who present with a tumour. The principles of investigation are shown in Box 20.2. The choice of test is often pragmatic, taking local access to reliable sampling facilities and laboratory measurements into account. Endocrine diseases present in many different ways and to clinicians in many different disciplines. Classical syndromes are described in relation to individual glands in the following sections. Often, however, the presentation is with non-specific symptoms (Box 20.3) or with asymptomatic biochemical abnormalities. In addition, endocrine diseases are encountered in the differential diagnosis of common complaints discussed in other chapters of this book, including electrolyte abnormalities (Ch. 16), hypertension (Ch. 18), obesity (Ch. 5) and osteoporosis (Ch. 25). Although diseases of the adrenal glands, hypothalamus and pituitary are relatively rare, their diagnosis often relies on astute clinical observation in a patient with non-specific complaints, so it is important that clinicians are familiar with their key features. Diseases of the thyroid predominantly affect females and are common, occurring in about 5% of the population. The thyroid axis is involved in the regulation of cellular differentiation and metabolism in virtually all nucleated cells, so that disorders of thyroid function have diverse manifestations. Structural diseases of the thyroid gland, such as goitre, commonly occur in patients with normal thyroid function. Diseases of the thyroid are summarised in Box 20.4. Thyroid physiology is illustrated in Figure 20.3. The parafollicular C cells secrete calcitonin, which is of no apparent physiological significance in humans. The follicular epithelial cells synthesise thyroid hormones by incorporating iodine into the amino acid tyrosine on the surface of thyroglobulin (Tg), a protein secreted into the colloid of the follicle. Iodide is a key substrate for thyroid hormone synthesis; a dietary intake in excess of 100 µg/day is required to maintain thyroid function in adults. The thyroid secretes predominantly thyroxine (T4) and only a small amount of triiodothyronine (T3); approximately 85% of T3 in blood is produced from T4 by a family of monodeiodinase enzymes which are active in many tissues, including liver, muscle, heart and kidney. Selenium is an integral component of these monodeiodinases. T4 can be regarded as a pro-hormone, since it has a longer half-life in blood than T3 (approximately 1 week compared with approximately 18 hours), and binds and activates thyroid hormone receptors less effectively than T3. T4 can also be converted to the inactive metabolite, reverse T3. Production of T3 and T4 in the thyroid is stimulated by thyrotrophin (thyroid-stimulating hormone, TSH), a glycoprotein released from the thyrotroph cells of the anterior pituitary in response to the hypothalamic tripeptide, thyrotrophin-releasing hormone (TRH). A circadian rhythm of TSH secretion can be demonstrated with a peak at 0100 hrs and trough at 1100 hrs, but the variation is small so that thyroid function can be assessed reliably from a single blood sample taken at any time of day and does not usually require any dynamic stimulation or suppression tests. There is a negative feedback of thyroid hormones on the hypothalamus and pituitary such that in thyrotoxicosis, when plasma concentrations of T3 and T4 are raised, TSH secretion is suppressed. Conversely, in hypothyroidism due to disease of the thyroid gland, low T3 and T4 are associated with high circulating TSH levels. The anterior pituitary is very sensitive to minor changes in thyroid hormone levels within the reference range. Although the reference range for free T4 is 9–21 pmol/L (700–1632 pg/dL), a rise or fall of 5 pmol/L in an individual in whom the level is usually 15 pmol/L would be associated on the one hand with undetectable TSH, and on the other hand with a raised TSH. For this reason, TSH is usually regarded as the most useful investigation of thyroid function. However, interpretation of TSH values without considering thyroid hormone levels may be misleading in patients with pituitary disease (see Box 20.58, p. 787). Moreover, TSH may take several weeks to ‘catch up’ with T4 and T3 levels, for example, when prolonged suppression of TSH in thyrotoxicosis is relieved by antithyroid therapy. Heterophilic antibodies can also interfere with the TSH assay and cause a spuriously high measurement. Common patterns of abnormal thyroid function test results and their interpretation are shown in Box 20.5. Other modalities commonly employed in the investigation of thyroid disease include measurement of antibodies against the TSH receptor or other thyroid antigens (see Box 20.8, p. 741), radioisotope imaging, fine needle aspiration biopsy and ultrasound. Their use is described below. Thyrotoxicosis describes a constellation of clinical features arising from elevated circulating levels of thyroid hormone. The most common causes are Graves’ disease, multinodular goitre and autonomously functioning thyroid nodules (toxic adenoma) (Box 20.6). Thyroiditis is more common in parts of the world where relevant viral infections occur, such as North America. The clinical manifestations of thyrotoxicosis are shown in Box 20.7 and an approach to differential diagnosis is given in Figure 20.4. The most common symptoms are weight loss with a normal or increased appetite, heat intolerance, palpitations, tremor and irritability. Tachycardia, palmar erythema and lid lag are common signs. Not all patients have a palpable goitre, but experienced clinicians can discriminate the diffuse soft goitre of Graves’ disease from the irregular enlargement of a multinodular goitre. All causes of thyrotoxicosis can cause lid retraction and lid lag, due to potentiation of sympathetic innervation of the levator palpebrae muscles, but only Graves’ disease causes other features of ophthalmopathy, including periorbital oedema, conjunctival irritation, exophthalmos and diplopia. Pretibial myxoedema (p. 751) and the rare thyroid acropachy (a periosteal hypertrophy, indistinguishable from finger clubbing) are also specific to Graves’ disease. The first-line investigations are serum T3, T4 and TSH. If abnormal values are found, the tests should be repeated and the abnormality confirmed in view of the likely need for prolonged medical treatment or destructive therapy. In most patients, serum T3 and T4 are both elevated, but T4 is in the upper part of the reference range and T3 raised (T3 toxicosis) in about 5%. Serum TSH is undetectable in primary thyrotoxicosis, but values can be raised in the very rare syndrome of secondary thyrotoxicosis caused by a TSH-producing pituitary adenoma. When biochemical thyrotoxicosis has been confirmed, further investigations should be undertaken to determine the underlying cause, including measurement of TSH receptor antibodies (TRAb, elevated in Graves’ disease, Box 20.8) and isotope scanning, as shown in Figure 20.4. Other non-specific abnormalities are common (Box 20.9). An ECG may demonstrate sinus tachycardia or atrial fibrillation. Radio-iodine uptake tests measure the proportion of isotope that is trapped in the whole gland, but have been largely superseded by 99mtechnetium scintigraphy scans, which also indicate trapping, are quicker to perform with a lower dose of radioactivity, and provide a higher-resolution image. In low-uptake thyrotoxicosis, the cause is usually a transient thyroiditis (p. 751). Occasionally, patients induce ‘factitious thyrotoxicosis’ by consuming excessive amounts of a thyroid hormone preparation, most often levothyroxine. The exogenous thyroxine suppresses pituitary TSH secretion and hence iodine uptake, serum thyroglobulin and release of endogenous thyroid hormones. The T4:T3 ratio (typically 30 : 1 in conventional thyrotoxicosis) is increased to above 70 : 1 because circulating T3 in factitious thyrotoxicosis is derived exclusively from the peripheral monodeiodination of T4 and not from thyroid secretion. The combination of negligible iodine uptake, high T4:T3 ratio and a low or undetectable thyroglobulin is diagnostic. Atrial fibrillation occurs in about 10% of patients with thyrotoxicosis. The incidence increases with age, so that almost half of all males with thyrotoxicosis over the age of 60 are affected. Moreover, subclinical thyrotoxicosis (p. 745) is a risk factor for atrial fibrillation. Characteristically, the ventricular rate is little influenced by digoxin, but responds to the addition of a β-blocker. Thromboembolic vascular complications are particularly common in thyrotoxic atrial fibrillation so that anticoagulation with warfarin is required, unless contraindicated. Once thyroid hormone and TSH concentrations have been returned to normal, atrial fibrillation will spontaneously revert to sinus rhythm in about 50% of patients, but cardioversion may be required in the remainder. Patients should be rehydrated and given propranolol, either orally (80 mg 4 times daily) or intravenously (1–5 mg 4 times daily). Sodium ipodate (500 mg per day orally) will restore serum T3 levels to normal in 48–72 hours. This is a radiographic contrast medium which not only inhibits the release of thyroid hormones, but also reduces the conversion of T4 to T3 and is, therefore, more effective than potassium iodide or Lugol’s solution. Dexamethasone (2 mg 4 times daily) and amiodarone have similar effects. Oral carbimazole 40–60 mg daily (p. 748) should be given to inhibit the synthesis of new thyroid hormone. If the patient is unconscious or uncooperative, carbimazole can be administered rectally with good effect, but no preparation is available for parenteral use. After 10–14 days the patient can usually be maintained on carbimazole alone. Hypothyroidism is a common condition with various causes (Box 20.10), but autoimmune disease (Hashimoto’s thyroiditis) and thyroid failure following 131I or surgical treatment of thyrotoxicosis account for over 90% of cases, except in areas where iodine deficiency is endemic. Women are affected approximately six times more frequently than men. The clinical presentation depends on the duration and severity of the hypothyroidism. Those in whom complete thyroid failure has developed insidiously over months or years may present with many of the clinical features listed in Box 20.7. A consequence of prolonged hypothyroidism is the infiltration of many body tissues by the mucopolysaccharides, hyaluronic acid and chondroitin sulphate, resulting in a low-pitched voice, poor hearing, slurred speech due to a large tongue, and compression of the median nerve at the wrist (carpal tunnel syndrome). Infiltration of the dermis gives rise to non-pitting oedema (myxoedema), which is most marked in the skin of the hands, feet and eyelids. The resultant periorbital puffiness is often striking and may be combined with facial pallor due to vasoconstriction and anaemia, or a lemon-yellow tint to the skin caused by carotenaemia, along with purplish lips and malar flush. Most cases of hypothyroidism are not clinically obvious, however, and a high index of suspicion needs to be maintained so that the diagnosis is not overlooked in individuals complaining of non-specific symptoms such as tiredness, weight gain, depression or carpal tunnel syndrome. The key discriminatory features in the history and examination are highlighted in Figure 20.5. Care must be taken to identify patients with transient hypothyroidism, in whom life-long levothyroxine therapy is inappropriate. This is often observed during the first 6 months after subtotal thyroidectomy or 131I treatment of Graves’ disease, in the post-thyrotoxic phase of subacute thyroiditis and in post-partum thyroiditis. In these conditions, levothyroxine treatment is not always necessary, as the patient may be asymptomatic during the short period of thyroid failure. In the vast majority of cases, hypothyroidism results from an intrinsic disorder of the thyroid gland (primary hypothyroidism). In this situation, serum T4 is low and TSH is elevated, usually in excess of 20 mU/L. Measurements of serum T3 are unhelpful since they do not discriminate reliably between euthyroidism and hypothyroidism. Secondary hypothyroidism is rare and is caused by failure of TSH secretion in an individual with hypothalamic or anterior pituitary disease. Other non-specific abnormalities are shown in Box 20.9. In severe, prolonged hypothyroidism, the electrocardiogram (ECG) classically demonstrates sinus bradycardia with low-voltage complexes and ST segment and T-wave abnormalities. Measurement of thyroid peroxidase antibodies is helpful but further investigations are rarely required (see Fig. 20.5). Treatment is with levothyroxine replacement. It is customary to start with a low dose of 50 µg per day for 3 weeks, increasing thereafter to 100 µg per day for a further 3 weeks and finally to a maintenance dose of 100–150 µg per day. In younger patients, it is safe to initiate levothyroxine at a higher dose (for example, 100 µg per day), to allow a more rapid normalisation of thyroid hormone levels. Levothyroxine has a half-life of 7 days so it should always be taken as a single daily dose and at least 6 weeks should pass before repeating thyroid function tests and adjusting the dose, usually by 25 µg per day. Patients feel better within 2–3 weeks. Reduction in weight and periorbital puffiness occurs quickly, but the restoration of skin and hair texture and resolution of any effusions may take 3–6 months. As illustrated in Figure 20.5, most patients do not require specialist review but will require life-long levothyroxine therapy. The dose of levothyroxine should be adjusted to maintain serum TSH within the reference range. To achieve this, serum T4 often needs to be in the upper part of the reference range or even slightly raised, because the T3 required for receptor activation is derived exclusively from conversion of T4 within the target tissues, without the usual contribution from thyroid secretion. Some physicians advocate combined replacement with T4 and T3 or preparations of animal thyroid extract, but this approach remains controversial and is not supported by robust evidence. Some patients remain symptomatic despite normalisation of TSH and may wish to take extra levothyroxine, which suppresses TSH. However, suppressed TSH is a risk factor for osteoporosis and atrial fibrillation (p. 745; subclinical thyrotoxicosis), so this approach cannot be recommended. It is important to measure thyroid function every 1–2 years once the dose of levothyroxine is stabilised. This encourages patient compliance with therapy and allows adjustment for variable underlying thyroid activity and other changes in levothyroxine requirements (Box 20.11). Some patients have a persistent elevation of serum TSH despite an ostensibly adequate replacement dose of levothyroxine; most commonly, this is a consequence of suboptimal compliance with therapy. There may be differences in bioavailability between the numerous generic preparations of levothyroxine and so, if an individual is experiencing marked changes in serum TSH despite optimal compliance, the prescription of a branded preparation of levothyroxine could be considered. Levothyroxine absorption is maximal when the medication is taken before bed and may be further optimised by taking a vitamin C supplement.
Endocrine disease
Clinical examination in endocrine disease
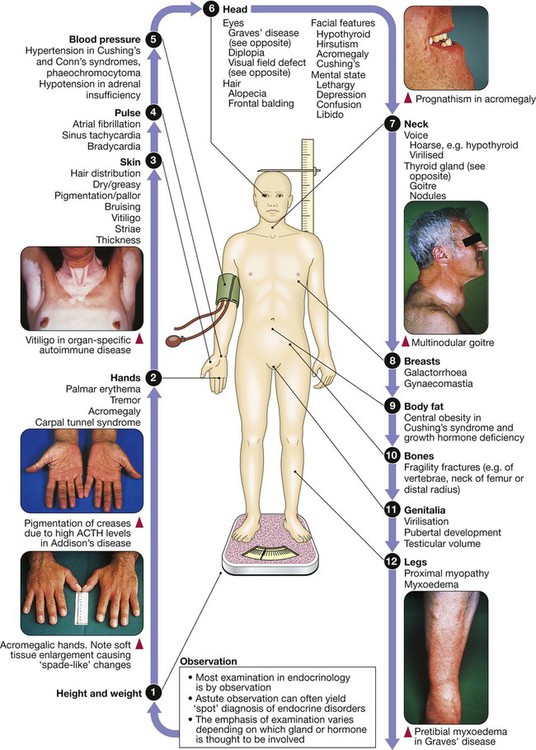
An overview of endocrinology
Functional anatomy and physiology
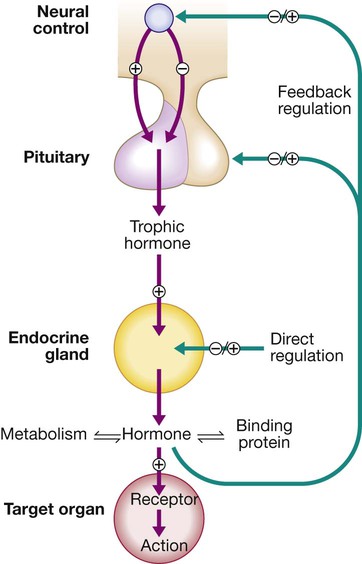
Regulation by negative feedback and direct control is shown, along with the equilibrium between active circulating free hormone and bound or metabolised hormone.
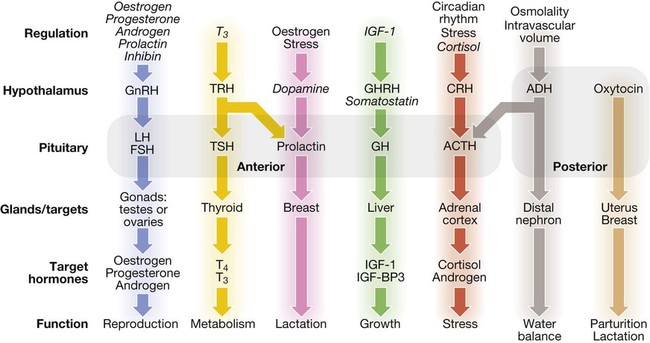
Some major endocrine glands are not controlled by the pituitary. These include the parathyroid glands (regulated by calcium concentrations, p. 766), the adrenal zona glomerulosa (regulated by the renin–angiotensin system, p. 771) and the endocrine pancreas (Ch. 21). Italics show negative regulation. (ACTH = adrenocorticotrophic hormone; ADH = antidiuretic hormone, arginine vasopressin; CRH = corticotrophin-releasing hormone; FSH = follicle-stimulating hormone; GH = growth hormone; GHRH = growth hormone-releasing hormone; GnRH = gonadotrophin-releasing hormone; IGF-1 = insulin-like growth factor-1; IGF-BP3 = IGF-binding protein-3; LH = luteinising hormone: T3 = triiodothyronine; T4 = thyroxine; TRH = thyrotrophin-releasing hormone; TSH = thyroid-stimulating hormone).
Endocrine pathology
Investigation of endocrine disease
Presenting problems in endocrine disease
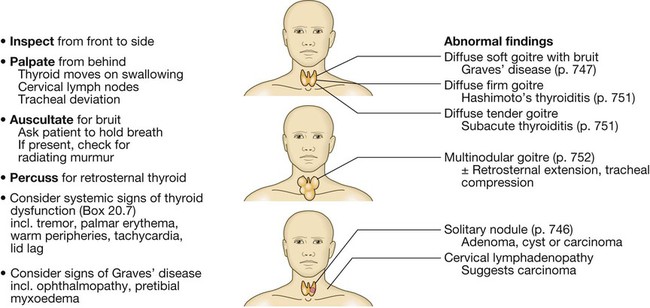
The thyroid gland
Functional anatomy, physiology and investigations
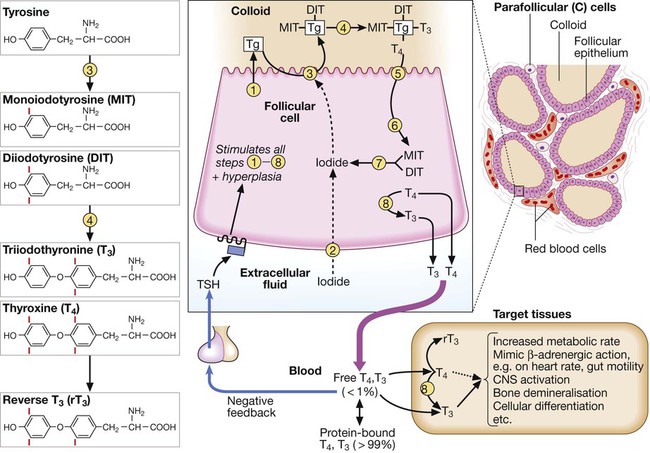
(1) Thyroglobulin (Tg) is synthesised and secreted into the colloid of the follicle. (2) Inorganic iodide (I−) is actively transported into the follicular cell (‘trapping’). (3) Iodide is transported on to the colloidal surface by a transporter (pendrin, defective in Pendred’s syndrome) and ‘organified’ by the thyroid peroxidase enzyme, which incorporates it into the amino acid tyrosine on the surface of Tg to form monoiodotyrosine (MIT) and diiodotyrosine (DIT). (4) Iodinated tyrosines couple to form T3 and T4. (5) Tg is endocytosed. (6) Tg is cleaved by proteolysis to free the iodinated tyrosine and thyroid hormones. (7) Iodinated tyrosine is dehalogenated to recycle the iodide. (8) T4 is converted to T3 by 5′-monodeiodinase.
Presenting problems in thyroid disease
Thyrotoxicosis
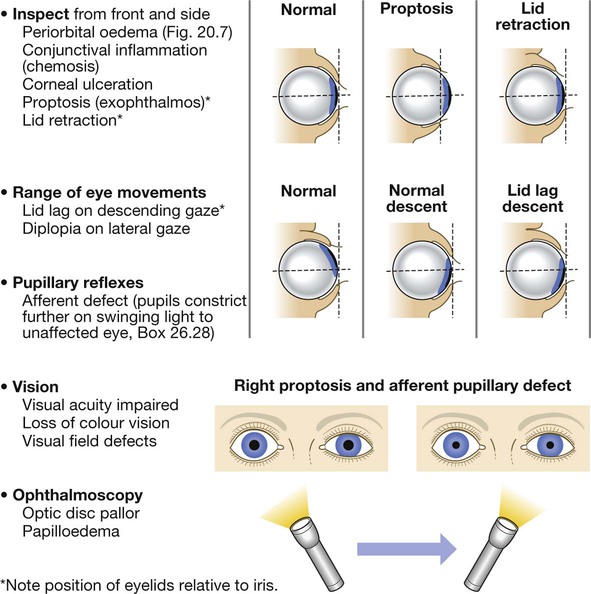
Clinical assessment
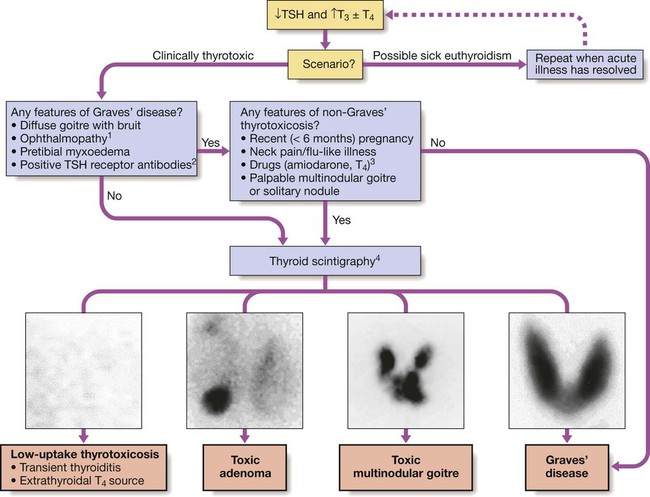
(1) Graves’ ophthalmopathy refers to clinical features of exophthalmos and periorbital and conjunctival oedema, not simply the lid lag and lid retraction which can occur in all forms of thyrotoxicosis. (2) TSH receptor antibodies are very rare in patients without autoimmune thyroid disease, but only occur in 80–95% of patients with Graves’ disease; a positive test is therefore confirmatory, but a negative test does not exclude Graves’ disease. Other thyroid antibodies (e.g. anti-peroxidase and anti-thyroglobulin antibodies) are unhelpful in the differential diagnosis since they occur frequently in the population and are found with several of the disorders which cause thyrotoxicosis. (3) Scintigraphy is not necessary in most cases of drug-induced thyrotoxicosis. (4) 99mTechnetium pertechnetate scans of patients with thyrotoxicosis. In low-uptake thyrotoxicosis, most commonly due to a viral, post-partum or iodine-induced thyroiditis, there is negligible isotope detected in the region of the thyroid, although uptake is apparent in nearby salivary glands (not shown here). In a toxic adenoma there is lack of uptake of isotope by the rest of the thyroid gland due to suppression of serum TSH. In multinodular goitre there is relatively low, patchy uptake within the nodules; such an appearance is not always associated with a palpable thyroid. In Graves’ disease there is diffuse uptake of isotope.
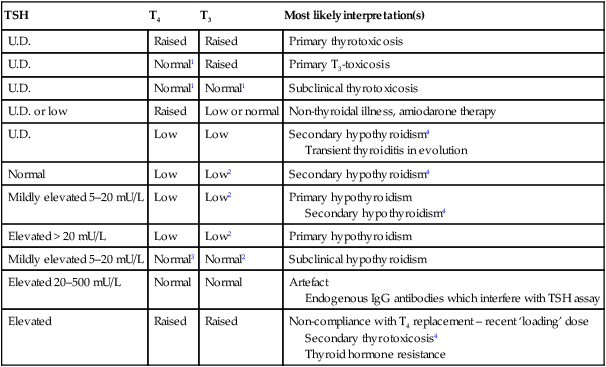
Investigations
Management
Atrial fibrillation in thyrotoxicosis
Thyrotoxic crisis (‘thyroid storm’)
Hypothyroidism
Clinical assessment
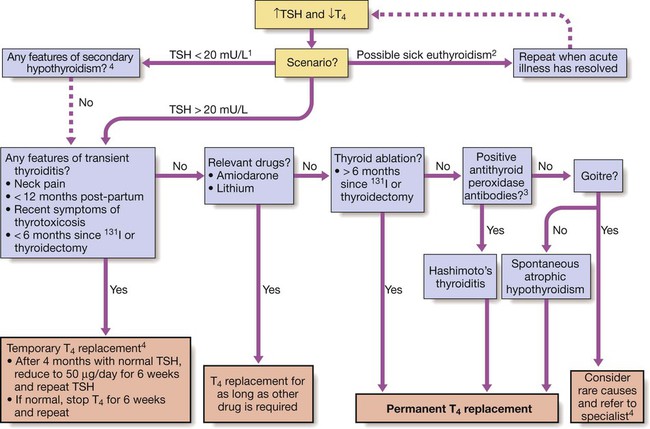
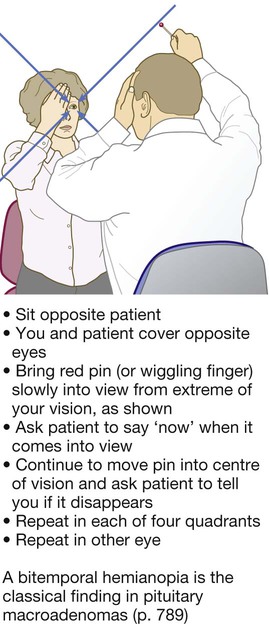
This scheme ignores congenital causes of hypothyroidism (see Box 20.10), such as thyroid aplasia and dyshormonogenesis (associated with nerve deafness in Pendred’s syndrome), which are usually diagnosed in childhood. (1) Immunoreactive TSH may be detected at normal or even modestly elevated levels in patients with pituitary failure; unless T4 is only marginally low, TSH should be > 20 mU/L to confirm the diagnosis of primary hypothyroidism. (2) The usual abnormality in sick euthyroidism is a low TSH but any pattern can occur. (3) Thyroid peroxidase antibodies are highly sensitive but not very specific for autoimmune thyroid disease (see Boxes 20.8 and 20.10). (4) Specialist advice is most appropriate where indicated. Secondary hypothyroidism is rare, but is suggested by deficiency of pituitary hormones or by clinical features of pituitary tumour such as headache or visual field defect (p. 789). Rare causes of hypothyroidism with goitre include dyshormonogenesis and infiltration of the thyroid (see Box 20.10).
Investigations
Management