CHAPTER 21 Secretory Membrane System and Golgi Apparatus
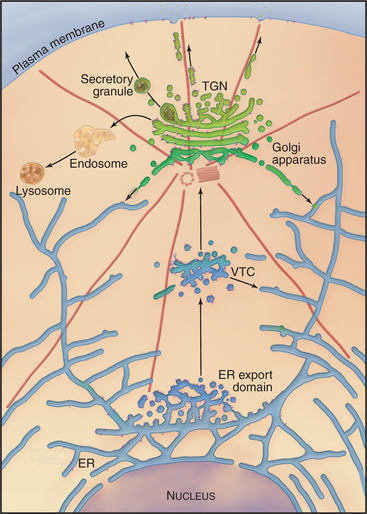
Eukaryotic cells transport newly synthesized proteins destined for the extracellular space, the plasma membrane, or the endocytic/lysosomal system through a series of functionally distinct, membrane-bound compartments, including the endoplasmic reticulum (ER), Golgi apparatus, and vesicular transport intermediates. This is the secretory membrane system (Fig. 21-1), which allows eukaryotic cells to perform three major functions: (1) distribute proteins and lipids synthesized in the ER to the cell surface and other cellular sites, (2) modify and/or store protein and lipid molecules after their export from the ER, and (3) generate and maintain the unique identity and function of the ER, Golgi apparatus, and plasma membrane. This chapter describes how the secretory membrane system is organized and operates to fulfill these functions. It also provides a detailed description of the Golgi apparatus whose conserved features are central for the operation of the secretory membrane system.
Overview of the Secretory Membrane System
Newly synthesized transmembrane and lumenal proteins transported through the secretory system are called cargo. These include lumenal proteins destined to be stored within a compartment or secreted to the cell exterior, as well as transmembrane proteins that are retained in a particular compartment (e.g., Golgi processing enzymes), delivered to the plasma membrane, or recycled among compartments (e.g., transport machinery). Transfer of cargo molecules through the secretory system begins with their cotranslational insertion into or across the ER bilayer (see Fig. 20-7). The cargo molecules are next folded and assembled into forms that can be sorted and concentrated within membrane-bound transport intermediates (called vesicular tubular carriers [VTCs]) destined for the Golgi apparatus. Once packaged into and transported by such a carrier, cargo enters the Golgi apparatus, which serves as the central processing and sorting station in the secretory membrane system. Within the Golgi apparatus, numerous enzymes modify the cargo molecules by trimming or elongating the cargo’s glycan side chains or cleaving its polypeptides. Processed cargo is then sorted into membrane-bound carriers that bud out from the Golgi apparatus and move to the plasma membrane, to the endosome/lysosomal system, or back to the ER. In specialized cell types, the Golgi apparatus can sort certain classes of cargo into secretory granules (for storage and later release to the cell exterior in response to specific stimuli) or give rise to transport carriers that target to different polarized plasma membrane domains.
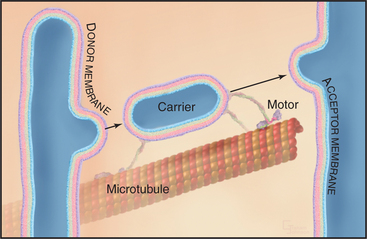
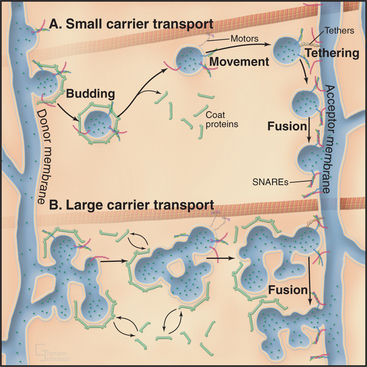
Membrane-enclosed carriers mediate transport within the secretory membrane system (Fig. 21-2). Carriers are shaped as tubules, vesicles, or larger structures. The carriers are too large to diffuse freely in the crowded cytoplasm but are transported over long distances along microtubules or actin filaments by molecular motor proteins. Each carrier selects certain types of cargo before budding from a donor compartment and fuses only with an appropriate target membrane. Molecular markers on the cytoplasmic surface of the carrier, as well as on the acceptor membrane, steer the carrier through the cytoplasm and ensure that it fuses only with the correct target compartment. The carriers continuously shuttle among ER, Golgi apparatus, and plasma membranes, enabling cargo to be distributed to its appropriate target organelle.
During transport of a carrier, the relative orientation (called topology) of lipid and protein in the membrane bilayer, established during synthesis in the ER, is maintained (Fig. 21-2). Hence, one side of the membrane always faces the cytoplasm. The other side initially faces the lumen of the ER. This side remains inside each membrane compartment along the secretory pathway but is exposed on the cell surface if the carrier fuses with the plasma membrane. Selection of proteins and lipids by a carrier, budding of the carrier, and subsequent fusion of the carrier with an acceptor compartment all also occur without leakage of contents from the carrier or the donor and target compartments.
The flow of cargo and lipid forward through the secretory system toward the plasma membrane (anterograde traffic) is balanced by selective retrograde traffic of cargo and lipids back toward the ER (Fig. 21-1). Retrograde traffic allows proteins and lipids involved in membrane transport and fusion to be retrieved for repeated use. Retrograde traffic also returns proteins that have been inadvertently carried forward through the secretory system so they can be redirected to their proper destination. Both anterograde and retrograde flows of membrane within the secretory system are necessary for the ER, Golgi apparatus, and plasma membrane to generate and maintain their distinct functional and morphologic identities.
Advantages of the Secretory Membrane System
A third advantage relates to the differentiation of the plasma membrane. Prokaryotic cells synthesize their proteins at the plasma membrane, so they must keep this surface enriched in loosely packed glycerophospholipids that are pliable enough that newly synthesized proteins can enter into and fold in a hydrophobic environment. Consequently, prokaryotic cells secrete a rigid cell wall as a protective barrier to the outside. In eukaryotes, concentrating protein synthesis in the ER frees the plasma membrane to become enriched in lipids such as cholesterol and sphingolipids that can arrange into highly ordered, flexible arrays. The ordered, flexible arrays of cholesterol and sphingolipids in the plasma membrane provide mechanical stability and an impermeable barrier to water-soluble molecules. As a consequence, eukaryotic cells do not require a cell wall to survive (although some eukaryotes, such as plant and fungal cells, make cell walls) and can employ their plasma membrane in a wide range of functions, such as membrane protrusion for engulfing large extracellular objects (see Chapter 22) and for crawling (see Chapter 38).
Building and Maintaining the Secretory Membrane System
Protein Sorting by the Lipid Gradient across the Secretory Membrane System
A conserved feature of the secretory membrane system is the differential distribution of various classes of lipids along the pathway. These classes of lipids include glycerophospholipids (phosphoglycerides), sphingolipids (e.g., sphingomyelin and glycosphingolipids), and cholesterol (see Figs. 7-4 and 20-13). These lipids play a major role in the sorting of proteins within the secretory membrane system because of their immiscibility (i.e., the property of not mixing) in membranes with different lipid compositions. By not mixing with some lipids while mixing with others, these lipid classes form lateral lipid assemblies, termed microdomains, that can concentrate or exclude specific membrane proteins.
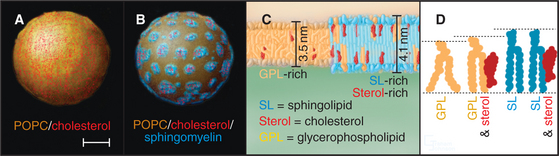
Studies using artificial membranes have demonstrated how lipid immiscibility allows a continuous lipid bilayer to self-organize into distinct lipid domains with unique lipid compositions and biophysical properties. A prime example is an artificial bilayer containing glycerophospholipids and cholesterol to which sphingolipid is added; after sphingolipid is added, the cholesterol and glycerophospholipids partition into distinct domains (Fig. 21-3A–B). Because of van der Waals attraction between the sphingolipid’s long, saturated hydrocarbon chain and cholesterol’s rigid, flat-cylindrical steroid backbone, the cholesterol and sphingolipids associate in the plane of the membrane, whereas glycerophospholipids, which have unsaturated, kinked hydrocarbon chains with much less affinity for cholesterol, are largely excluded from the cholesterol/sphingolipid domains. The domains enriched in cholesterol/sphingolipid are thicker than the surrounding membrane composed of shorter, unsaturated, kinked glycerophospholipids (Fig. 21-3C). Tension on the bilayer (i.e., from binding of proteins that bend or curve the membrane) enhances the tendency of lipids that have different physical properties to separate into distinct phases.
In addition to prompting separation of sphingolipids from glycerophospholipids, cholesterol can affect a bilayer composed of glycerophospholipids alone (Fig. 21-3 D). In this case, the cholesterol fills the space between the floppy hydrocarbon chains of glycerophospholipids in the bilayer. This forces the glycerophospholipids into a tighter alignment and increases the distance between their head groups. As a result, the bilayer becomes thicker, resembling the thickness of bilayers enriched in sphingomyelin alone or sphingomyelin plus cholesterol.
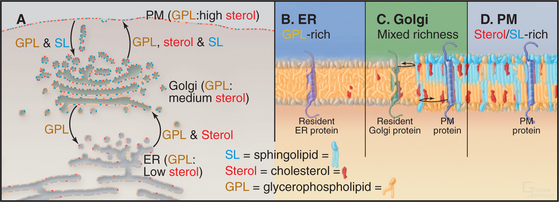
Sphingolipids (e.g., glycosphingolipids and sphingomyelin) are synthesized in the Golgi apparatus, while the ER produces cholesterol and glycerophospholipids. Synthesis of these lipids at two different sites, combined with the self-organizing capacity of sphingolipids, cholesterol, and glycerophospholipids, gives rise to a pattern of lipid circulation within the secretory system that plays important roles in membrane sorting (Fig. 21-4A). Newly synthesized cholesterol is continually removed from the ER and redistributed to the Golgi apparatus, where high affinity interactions with sphingolipids prevent it from returning to the ER. The association of cholesterol with sphingolipids in the Golgi apparatus, in turn, triggers the lateral differentiation of domains enriched in these lipids. Through the additional activity of protein-based sorting and trafficking machinery, these domains bud off the Golgi apparatus and move to the plasma membrane, redistributing sphingolipids and cholesterol to the cell surface.
The forward flow of cholesterol, sphingolipids, and glycerophospholipids toward the plasma membrane is balanced by selective retrograde flow. Glycerophospholipids transferred from the ER to the Golgi apparatus are recycled back to the ER. Similarly, sphingolipids delivered to the plasma membrane from the Golgi apparatus are returned to the Golgi apparatus. Cholesterol, in contrast, is not returned through these retrograde pathways to either the ER or the Golgi apparatus but enters and circulates within the endocytic pathway leading to lysosomes. This pattern of lipid circulation creates a gradient of cholesterol, sphingolipids, and glycerophospholipids across the secretory membrane system. Within this gradient, the ER has a low concentration of cholesterol (e.g., sterols) and sphingolipids, the Golgi apparatus has an intermediate concentration, and the plasma membrane has a high concentration (Fig. 21-4 A).
A second function of the lipid gradient is to promote sorting of transmembrane proteins within the secretory system. Each integral membrane protein seeks a lipid bilayer with a thickness that matches the lengths of its transmembrane segments (Fig. 21-4B–D). Because most transmembrane segments are stiff hydrophobic a-helices, it is energetically unfavorable to expose hydrophobic residues of a transmembrane polypeptide to the aqueous environment of the cytoplasm or vesicle lumen or to bury hydrophilic amino acids with the lipid acyl chains in the interior of the membrane. To avoid such hydrophobic mismatches, integral membrane proteins of the secretory system have evolved with transmembrane segments that are matched to the thickness of their target membranes. Hence, resident membrane proteins in the ER and Golgi apparatus typically have shorter transmembrane segments (around 15 amino acids) than do resident plasma membrane proteins (approximately 20 to 25 amino acids). Retention and/or transport of these proteins occurs because the lipid bilayers of carriers budding out from either the ER (toward the Golgi apparatus) or the Golgi apparatus (toward the plasma membrane) are thicker than the bilayers of the donor organelles. Only transmembrane proteins with transmembrane segments long enough to span this thickness enter such carriers.
This lipid-based protein sorting mechanism takes advantage of the lipid gradient established by the self-organizing properties of glycerophospholipids, cholesterol, and sphingolipids to sort and transport proteins within the secretory system. It is not, however, the only mechanism used by cells to organize and transport proteins along the secretory pathway. In addition, a complex protein-based machinery is relied on to bring far greater specificity and efficiency to these processes.
Protein-Based Machinery for Protein Sorting and Transport within the Secretory Membrane System
Sorting and transporting proteins within the secretory membrane system depend on several types of proteins (Fig. 21-5): Specialized “coats” help to generate both small and large transport carriers and sort proteins into them; motor proteins move carriers along the cytoskeleton; “tethering factors” attach carriers to the cytoskeleton and to their destination organelles prior to fusion; and fusion proteins mediate fusion of the carrier with an acceptor membrane. These components also associate with specific organelles, providing organelles with an identity that is both unique and dynamic. Many of the components are peripheral membrane proteins that lack transmembrane domains, so they must be recruited to the cytoplasmic surface of appropriate membranes by binding to either specific lipids, such as phosphoinositides, or to activated GTPases. Cells regulate the distributions of these organelle-specific lipids and GTPases. When infectious agents or stressful conditions disrupt these targeting molecules, secretory membrane trafficking can be disorganized and/or inhibited. The following sections describe the six major protein-based mechanisms that are used for sorting, transport, and fusion in the secretory membrane system.
Arf GTPases
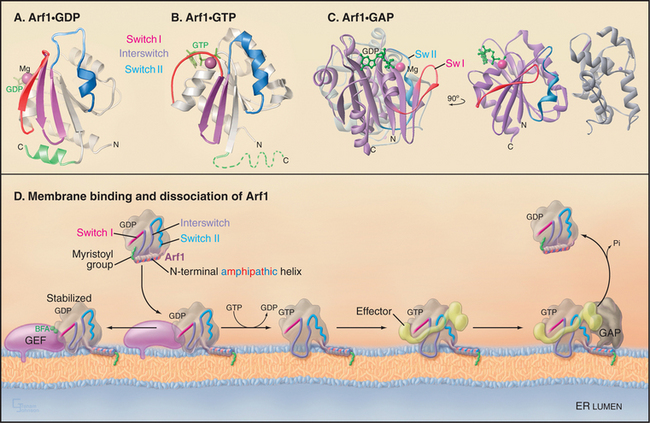
Like other GTPases (see Figs. 4-6 and 4-7), Arfs are molecular switches that alternate between a GTP-bound active form that interacts with effector targets and a GDP-bound inactive form that does not (Fig. 21-6). Active Arf GTPases associate with membranes, where-as inactive GTPases are cytoplasmic. Specific GTP exchange factors (GEFs) recruit Arf proteins to particular membrane surfaces and then catalyze the exchange of GDP for GTP. When associated with particular membranes active Arfs bind their effectors until a GTPase-activating protein (GAP) induces hydrolysis of GTP, reversing membrane association and effector binding. The distribution of GEFs on particular membranes determines the location of specific active Arfs. Similarly, the location of GAPs determines where each type of Arf is inactivated.
Activation of Arfs by exchange of GDP for GTP not only creates a binding site for target proteins (i.e., effectors) but also promotes interaction with the lipid bilayer. A myristoyl group covalently bound to the N-terminus of most Arfs allows them to interact transiently and nonspecifically with membranes. When a specific Arf-GEF on a membrane catalyzes the exchange of GDP for GTP, an amphipathic (hydrophobic on one side, hydrophilic on the other) N-terminal, a-helix is released from a hydrophobic pocket on the GTPase so that the hydrophobic side of the helix can interact with the bilayer (Fig. 21-6 D). The membrane-associated GEFs that are responsible for activating Arfs all contain an evolutionarily conserved domain (referred to as the Sec7 domain). Association of this domain with Arf1-GDP is stabilized in the presence of the toxic fungal metabolite brefeldin A (BFA [Fig. 21-6 D]). This prevents Arf1 conversion to its active, GTP-bound state and thereby blocks Arf1 activity, similar to that of a GDP-locked Arf1 mutant.
Arf GTPases of the secretory pathway, in particular Sar1 and Arf1, recruit to membranes many types of effector proteins. These include the coat protein complexes of COPII, COPI, and clathrin/adapters plus other effectors such as phospholipid modifiers (e.g., phospholipase D, a lipid metabolizing enzyme), phosphoinositides, and cytoskeletal components. The coat protein complexes assemble into large polymeric structures (called protein coats) at the cytoplasmic surface of ER, pre-Golgi, and Golgi membranes, from which they sort cargo and promote the budding of transport carriers. The other Arf effectors play roles in differentiating the membrane environment of these carriers and enabling them to move to different locations within the cell. The four other mammalian Arf proteins (Arfs 2 to 6) regulate vesicle formation at other locales in the exocytic and endocytic pathways.
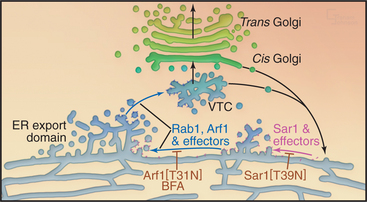
Sar1 assembles the COPII coat complex that is involved in differentiating ER export domains, which are the sites from which transport carriers bud out from the ER. Arf1, by contrast, assembles the COPI coat complex that is involved in the creation of retrograde transport carriers that bud from pre-Golgi and Golgi structures. Arf1 also recruits the clathrin/adapter coat complexes that are involved in budding of transport carriers from the Golgi en route to the endosome/lysosomal system. Disruption of the GTPase cycles of either Sar1 or Arf1 has dramatic consequences for secretory transport and the organization of the secretory pathway (Fig. 21-14). When the GTPase cycles of Sar1 or Arf1 are disrupted, the Golgi apparatus disassembles, and Golgi enzymes return to the ER or to ER exit sites with all secretory transport out of the ER inhibited.
The COPII Coat
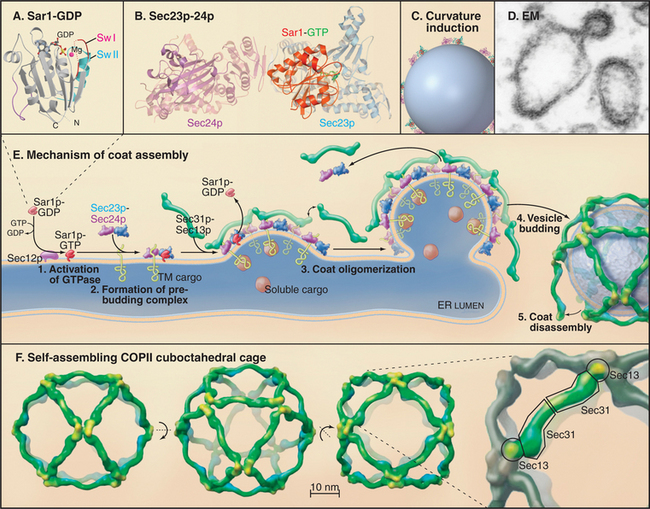
The COPII coat complex (Fig. 21-7) is essential for sorting and trafficking secretory cargo out of the ER. It consists of Sar1p GTPase, the Sec23p˙Sec24p subcomplex, and the Sec13p˙Sec31p subcomplex. These components self-assemble into a polymeric, two-dimensional scaffold (called a coat) that then collects specific types of cargo. The intrinsic curvature of the coat promotes the formation of membrane buds that are capable of pinching off the membrane as coated vesicles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree