Established indications
Rule out metastasis of extrarenal primary involving kidney
Rule out lymphoma involving kidney
Rule out renal mass caused by a benign condition such as infection
Patients with unresectable tumor
Patients with surgical comorbidities
Emerging indications
Distinguish between benign and malignant tumors for small renal masses
Define the histologic subtypes of primary renal neoplasms for risk assessment and therapy selection
Patients with a renal mass considered for percutaneous ablation
Safety
With guidance provided by modern imaging modalities and improved intervention techniques, percutaneous needle biopsy of renal masses is a safe procedure today [10, 11]. The most frequent complication reported has been bleeding, which is usually subclinical and self-limiting. Major bleeding that requires a blood transfusion has been rare and often can be minimized by correcting coagulation abnormalities and controlling hypertension. Hematuria can occur in 5–7 % of cases, but is generally also self-limited. Very rare events such as pseudoaneurysm and arteriovenous fistula formation may cause persistent bleeding and hematuria, but can usually be managed by embolization if needed. Pneumothorax is also rare and can be best avoided using a subcostal approach.
Tumor seeding along the needle track has been a worrisome concern of renal biopsy . However, the risk of seeding has been estimated to be less than 0.01 % [12]. There have been only few such events reported in the literature, suggesting that it is a very rare complication of renal needle biopsy. A recent case report emphasizes the importance of imaging surveillance of the needle tract used by percutaneous biopsy or ablation [13].
Accuracy
The sensitivity and diagnostic accuracy of renal needle biopsy has also been improved significantly in recent years. In core biopsy series published in the last decade, the sensitivity of renal biopsy has ranged from over 70 to 100 %, while the diagnostic accuracy has often been superior to 90 % [10, 11]. The enhanced sensitivity and accuracy are likely consequences of better tumor visualization, improved biopsy technique, increased experience with renal core biopsy interpretation, as well as advances in ancillary studies utilized in pathologic evaluation.
On the other hand, false-negative results can happen because of inappropriate needle placement or obtaining necrotic or scant diagnostic tissue. Therefore, an absence of malignant cells from a biopsy does not necessarily exclude the presence of a malignancy and should be interpreted with caution. In this regard, small masses (≤ 3 cm) may have higher false-negative rates, due to inaccurate targeting and/or insufficient diagnostic material obtained, which can be reduced by repeat biopsies and a high level of experience in the procedure operator and pathologists [14].Core biopsies and FNA may have complementary roles as shown in some published series [15, 16]. Performing an onsite FNA assessment of sample adequacy is also likely to help enhance the yield of renal needle biopsies.
Histologic Interpretation and Ancillary Studies
Consistent with the current clinical indications for renal needle biopsies, entities that may be encountered for histologic interpretation include a wide spectrum of neoplastic and non-neoplastic diseases. In addition to renal cell neoplasms, the differential considerations should also comprise various primary renal tumors such as mixed mesenchymal and epithelial tumors , mesenchymal tumors, metanephric tumors, and neuroendocrine tumors, as well as metastatic malignancies to the kidney, tumors arising from adjacent organ sites (e.g., adrenal gland, urinary bladder, and retroperitoneum), primary or secondary lymphomas, and mass-forming benign conditions such as infection. Similar to resection specimens, the interpretation of renal core biopsy relies on a careful examination of both the cytologic and architectural features of sampled tissue. However, renal core biopsies typically only reveal limited diagnostic material, which may make the recognition of architectural patterns incomplete or inconclusive. Given the broad range of histologic features that can be seen in a renal core biopsy, a commonly utilized interpretation approach is to categorize the potential tumors/lesions based on certain cytologic features (e.g., clear vs. eosinophilic) and/or architectural patterns (e.g., papillary) that are discernible even in limited diagnostic material to help narrow down the number of entities considered in the differential diagnosis. While this is a very practical approach, one should be aware of the fact that a limited sampling by core biopsy can be misleading especially when only unusual histologic features are revealed. For difficult or ambiguous cases, ancillary studies such as a selected panel of immunohistochemical stains are often very helpful for establishing a correct diagnosis [17]. The main entities in some of these histologic categories as well as ancillary studies are briefly discussed below. The commonly utilized immunostains for differentiating primary epithelial neoplasms of the kidney are summarized in Table 33.2.
Table 33.2
Immunohistochemical stains commonly used in the differential diagnosis of primary epithelial tumors of the kidney
Clear cell RCC | Clear cell papillary RCC | Papillary RCC type 1 | Papillary RCC type 2 | Chromophobe RCC | Renal oncocytoma | MTSCC | Translocation RCC | Metanephric adenoma | Collecting duct carcinoma | Renal medullary carcinoma | Urothelial carcinoma | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Pax8/Pax2 | + | + | + | + | +/− | +/− | + | + | + | +/− | +/− | −/+ |
CA-IX (diffuse, membranous) | + | +a | – | – | – | – | – | −/+(focal) | – | – | – | −/+ |
CD10 | + | – | +(often luminal) | +/− | +/− | – | +/− | + | – | – | – | – |
CK7 | – | + | + | +/− | +/− | −/+ | + | – | −/+(focal) | −/+ | −/+ | + |
AMACR | – | – | + | +/− | – | – | + | – | – | −/+ | −/+ | −/+ |
CD117 | – | – | – | – | + | + | – | – | – | – | – | – |
TFE3/TFEB | – | – | – | – | – | – | – | + | – | – | – | – |
Cathepsin-K | – | – | – | – | – | – | – | + | – | – | – | – |
HMB-45 | – | – | – | – | – | – | – | −/+ | – | – | – | – |
Melan-A | – | – | – | – | – | – | – | −/+ | – | – | – | – |
INI1 (BAF47) | + | + | + | + | + | + | + | + | + | + | – | + |
34βE12 (HMWCK) | – | +/− | – | – | – | – | – | – | – | +/− | −/+ | + |
p63 | – | – | – | – | – | – | – | – | – | – | – | + |
GATA3 | – | – | – | – | – | – | – | – | – | – | – | + |
Clear Cell Cytology
Clear cell RCC is the most common type of RCC and comprises approximately 60 % of all renal cortical tumors. In a renal core biopsy mainly showing clear cell cytology, clear cell RCC is usually the top consideration among other differential diagnoses. Other tumors or tumor-like lesions that may exhibit clear cell cytology include clear cell papillary RCC, chromophobe RCC, TFE3/TFEB translocation-associated RCC, papillary RCC with focal clear cell areas, adrenal cortical tissue/tumor in an ectopic location or being mis-sampled by the biopsy procedure, angiomyolipoma with abundant clear cells, and foamy histiocyte-rich lesions such as xanthogranulomatous pyelonephritis [18]. The cytoplasm of clear cells in a clear cell RCC or clear cell papillary RCC is typically optically transparent, whereas the clear cytoplasm in a chromophobe RCC tends to show fine granular or fibrillary eosinophilic material. The areas of clear cells in a papillary RCC, more commonly type 1, often also exhibit cytoplasm with focal granular eosinophilia. The clear cells seen in adrenal cortical tissue/tumor have a uniform, vacuolated bubbly appearance, mimicking cells in sebaceous glands (Fig. 33.1a–d).
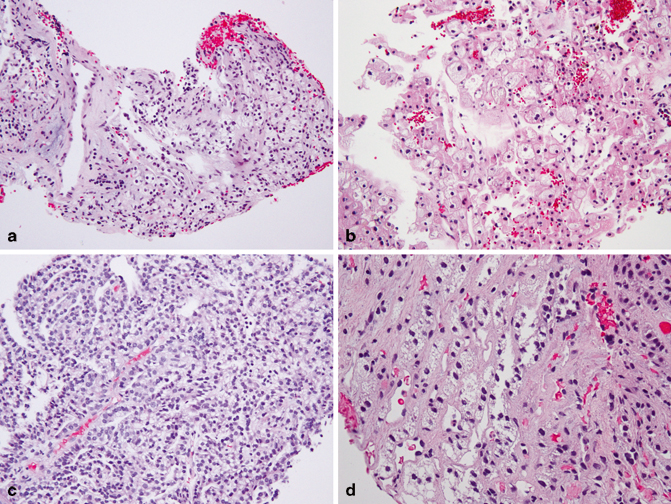

Fig. 33.1
Core biopsies of a clear cell RCC (a), chromophobe RCC (b), type 1 papillary RCC with clear cells (c), and adrenal cortical tissue (d). Note the quality of the clear cytoplasm varies in each case
Clear cell RCC typically comprises solid acini or nests of clear cells separated by delicate, intricately branching fibrovascular septa. Tubular, papillary/pseudopapillary, large alveolar, and solid sheet-like growth patterns can also be seen . Higher nuclear grade often shows a loose association with focal or marked cytoplasmic eosinophilia and certain architectures such as solid sheet-like, pseudopapillary, or large alveolar patterns. Hyalinization is a common finding in these tumors. In extreme examples, rare clusters of clear cells remaining in the hyalinized stroma can be easily missed, whereas the rich vasculature in the stroma can lead to misinterpretation as a vascular lesion. The classical appearance of clear cell RCC, even only present in a focal area, would strongly suggest this diagnosis. Diffuse, membranous staining of carbonic anhydrase IX (CA-IX) in non-necrotic areas is a useful feature to separate clear cell RCC from other primary renal cell neoplasms .
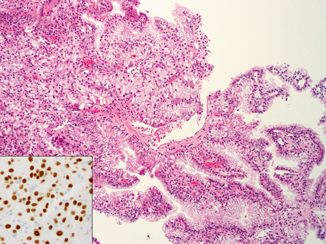
Clear cell papillary RCC is characterized by tumor cells with uniformly clear cytoplasm and low-grade nuclei that are arranged in a linear fashion, away from the basal aspect of the cells. Although this linear arrangement of nuclei may be less apparent in cells with minimal cytoplasm in areas showing collapsed tubular/acinar patterns (Fig. 33.2), an appreciation of such features even in focal areas would be sufficient to trigger a small panel of immunohistochemical studies to help distinguish them from a clear cell or papillary RCC. The clear cell papillary RCC is diffusely positive for CK7, negative for CD10 and AMACR, while showing a cup-shaped membranous staining pattern for CA-IX (absence of staining along the luminal border). Because of its overall indolent behavior, clear cell papillary RCC is an important diagnosis to be recognized on renal core biopsy, as patients may benefit from a more conservative management plan.
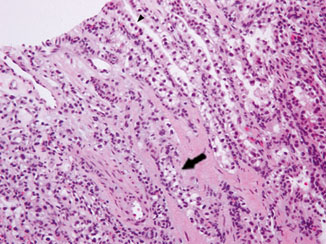

Fig. 33.2
Core biopsy of clear cell papillary RCC showing tubulopapillary growth and low-grade nuclei. Note the apparent linear arrangement of nuclei away from the basal aspect of tumor cells in some areas ( arrowhead). The feature is difficult to appreciate in the more collapsed area ( arrow)
Chromophobe RCC is composed of large polygonal cells with prominent cell borders. Besides the finely granular/reticulated cytoplasm, the wrinkled nuclei and perinuclear halos are helpful features that are distinct from a clear cell RCC. Although a portion of chromophobe RCCs are aggressive, stage by stage, they have a significantly better prognosis than clear cell RCCs .
Papillary RCC typically shows amphophilic or eosinophilic cytoplasm, but focal cytoplasmic clearing is not uncommon, particularly in type 1 papillary RCC . The main differential diagnoses are other tumors with clear cells and papillary or tubulopapillary architecture (see below for further discussion) .
MiTF family translocation-associated RCCs are defined by translocations involving MiTF/TFE family genes ( TFE3 or TFEB) and often demonstrate a wide range of histologic features. TFE3 translocation tumors often show abundant clear cytoplasm and high-grade nuclei and display papillary, alveolar, or solid growth. Admixed eosinophilic cells are also common. Psammoma bodies and cytoplasmic hyaline globules are frequently found. Some TFE3 translocation tumors can show lower nuclear grade and less cytoplasm. TFEB translocation tumors typically show biphasic morphology, comprising larger epithelioid cells and smaller cells clustered around basement membrane-like material. Both types of translocation tumors can show significant morphologic overlap with clear cell or other types of RCCs, and their diagnosis on core biopsy relies on immunohistochemical tests for TFE3/TFEB overexpression (Fig. 33.3) or fluorescence in situ hybridization (FISH) assays to confirm TFE3 or TFEB rearrangement. Cathepsin-K expression detected by immunohistochemistry has also been found to be a useful marker for TFEB tumors, TFE3 tumors with PRCC-TFE3 fusion, as well as alveolar soft part sarcomas [19]. FISH break-apart assays appear to have a higher sensitivity to detect TFE3/TFEB translocation than the TFE3/TFEB immunohistochemical staining and have revealed an expanding histologic spectrum for these tumors [20, 21].