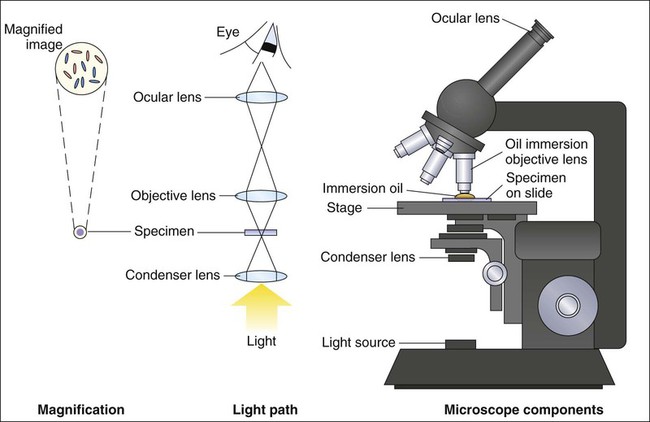
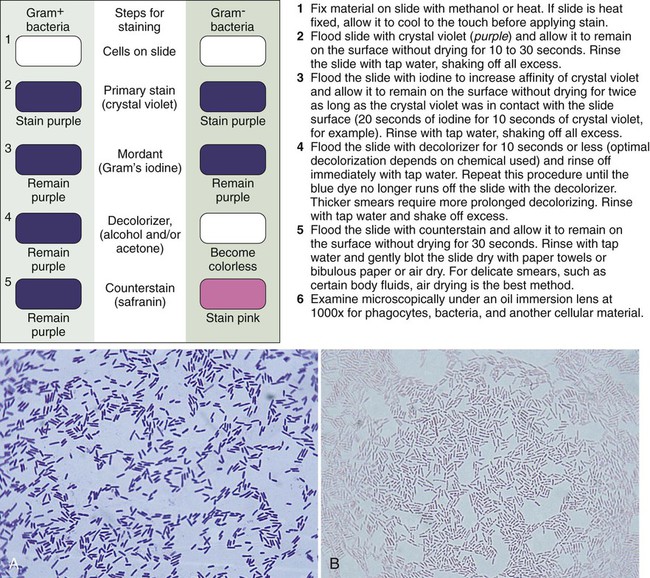
1. Explain the role of microscopy in the identification of etiologic agents including bacteria, fungi, viruses, and parasites. 2. List the four types of microscopy available for diagnostic evaluation, explain their basic principles, and list a clinical application for each. 3. Define the three main principles of light microscopy, magnification, resolution, and contrast. 4. List the staining techniques used to aid in the visualization of bacteria, explain the chemical principle and limitations for each, and provide an example of a clinical application for each stain. 5. Include the following stains: Gram stain, the Kinyoun stain, the Ziehl-Neelsen stain, the Calcofluor white stain, the Acridine orange stain, and the Auramine-Rhodamine stain. 6. Explain the chemical principle for fluorescent dyes in microscopy, and list two examples routinely used in the clinical laboratory. 1. Direct examination of patient specimens for the presence of etiologic agents 2. Growth and cultivation of the agents from these same specimens 3. Analysis of the cultivated organisms to establish their identification and other pertinent characteristics such as susceptibility to antimicrobial agents Microscopy is the most common method used both for the detection of microorganisms directly in clinical specimens and for the characterization of organisms grown in culture (Box 6-1). Microscopy is defined as the use of a microscope to magnify (i.e., visually enlarge) objects too small to be visualized with the naked eye so that their characteristics are readily observable. Because most infectious agents cannot be detected with the unaided eye, microscopy plays a pivotal role in the laboratory. Microscopes and microscopic methods vary, but only those of primary use in diagnostic microbiology are discussed. The method used to process patient specimens is dictated by the type and body source of specimen (see Part VII). Regardless of the method used, some portion of the specimen usually is reserved for microscopic examination. Specific stains or dyes applied to the specimens, combined with particular methods of microscopy, can detect etiologic agents in a rapid, relatively inexpensive, and productive way. Microscopy also plays a key role in the characterization of organisms that have been cultivated in the laboratory (for more information regarding cultivation of bacteria, see Chapter 7). The types of microorganisms to be detected, identified, and characterized determine the most appropriate types of microscopy to use. Table 6-1 outlines the four types of microscopy used in diagnostic microbiology and their relative utility for each of the four major types of infectious agents. Bright-field microscopy (also known as light microscopy) and fluorescence microscopy have the widest use and application within the clinical microbiology laboratory. Dark field and electron microscopes are not typically found within a clinical laboratory and are predominantly used in reference or research settings. Which microorganisms can be detected or identified by each microscopic method also depends on the methods used to highlight the microorganisms and their key characteristics. This enhancement is usually achieved using various dyes or stains. TABLE 6-1 Microscopy for Diagnostic Microbiology +, Commonly used; ±, limited use; –, rarely used. For light microscopy, visible light is passed through the specimen and then through a series of lenses that bend the light in a manner that results in magnification of the organisms present in the specimen (Figure 6-1). The total magnification achieved is the product of the lenses used. In most light microscopes, the objective lens, which is closest to the specimen, magnifies objects 100× (times), and the ocular lens, which is nearest the eye, magnifies 10×. Using these two lenses in combination, organisms in the specimen are magnified 1000× their actual size when viewed through the ocular lens. Objective lenses of lower magnification are available so that those of 10×, 20×, and 40× magnification power can provide total magnifications of 100×, 200×, and 400×, respectively. Magnification of 1000× allows for the visualization of fungi, most parasites, and most bacteria, but it is not sufficient for observing viruses, which require magnification of 100,000× or more (see Electron Microscopy in this chapter). The third key component to light microscopy is contrast, which is needed to make objects stand out from the background. Because microorganisms are essentially transparent, owing to their microscopic dimensions and high water content, they cannot be easily detected among the background materials and debris in patient specimens. Lack of contrast is also a problem for the microscopic examination of microorganisms grown in culture. Contrast is most commonly achieved by staining techniques that highlight organisms and allow them to be differentiated from one another and from background material and debris. In the absence of staining, the simplest way to improve contrast is to reduce the diameter of the microscope aperture diaphragm increasing contrast at the expense of the resolution. Setting the controls for bright field microscopy requires a procedure referred to as setting the Kohler illumination (see Procedure 6-1 on the Evolve site). Generally, specimen samples are placed on the slide using a swab that contains patient material or by using a pipette into which liquid specimen has been aspirated (Figure 6-2). Material to be stained is dropped (if liquid) or rolled (if on a swab) onto the surface of a clean, dry, glass slide. To avoid contamination of culture media, once a swab has touched the surface of a nonsterile slide, it should not be used for subsequently inoculating media. As listed in Table 6-1, light microscopy has applications for bacteria, fungi, and parasites. However, the stains used for these microbial groups differ extensively. Those primarily designed for examination of parasites and fungi by light microscopy are discussed in Chapters 47 and 60, respectively. The stains for microscopic examination of bacteria, the Gram stain and the acid-fast stains, are discussed in this chapter. Although modifications of the classic Gram stain that involve changes in reagents and timing exist, the principles and results are the same for all modifications. The classic Gram stain procedure entails fixing clinical material to the surface of the microscope slide, either by heating or by using methanol. Methanol fixation preserves the morphology of host cells, as well as bacteria, and is especially useful for examining bloody specimen material. Slides are overlaid with 95% methanol for 1 minute; the methanol is allowed to run off, and the slides are air-dried before staining. After fixation, the first step in the Gram stain is the application of the primary stain crystal violet. A mordant, Gram’s iodine, is applied after the crystal violet to chemically bond the alkaline dye to the bacterial cell wall. The decolorization step distinguishes gram-positive from gram-negative cells. After decolorization, organisms that stain gram-positive retain the crystal violet and those that are gram-negative are cleared of crystal violet. Addition of the counterstain safranin will stain the clear gram-negative bacteria pink or red (Figure 6-3). See Procedure 6-2 on the Evolve site for detailed methodology, expected results, and limitations.
Role of Microscopy
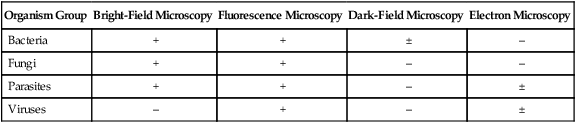
Organism Group
Bright-Field Microscopy
Fluorescence Microscopy
Dark-Field Microscopy
Electron Microscopy
Bacteria
+
+
±
–
Fungi
+
+
–
–
Parasites
+
+
–
±
Viruses
–
+
–
±
Bright-Field (Light) Microscopy
Principles of Light Microscopy
Magnification
Contrast
Staining Techniques for Light Microscopy
Smear Preparation
Gram Stain
Procedure Overview.