Chapter 13
Ribonucleic acids as drugs and drug targets
13.1 Introduction
As we have seen in the preceding chapters, most drugs target proteins, but some drugs act on ribonucleic acids (RNAs) instead. Among these, the currently most widely used ones are antibiotics that inhibit the bacterial ribosome (see slide 11.5). However, as more has been learned about the diverse and crucial roles of RNA in eukaryotic cell biology, novel RNA drug targets have emerged and become a focus of ongoing drug development efforts.
In this chapter, we will first have a closer look at some of the antibiotics that interact with the RNA of the bacterial ribosome, and then turn to some of the experimental strategies that target RNA molecules in human cells.
13.2 Antibiotics that inhibit the bacterial ribosome
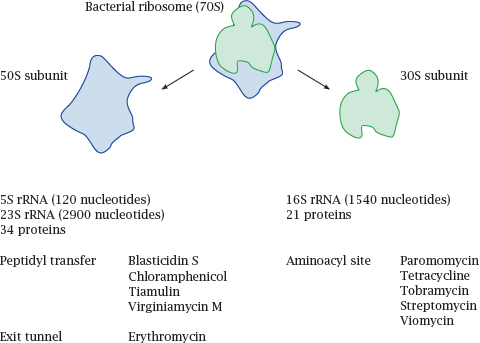
13.2.1 Some aminoglycoside antibiotics
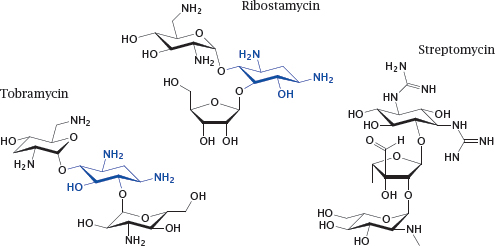
Notes: Aminoglycoside antibiotics are produced by Streptomyces species and related soil bacteria. As the name suggests, they are oligosaccharides substituted with primary or secondary amines and related functional groups. These antibiotics bind to the aminoacyl acceptor site in the small subunit of the bacterial ribosome.
Among the antibiotics shown here, ribostamycin is a “typical” aminoglycoside that belongs to the 4,5-disubstituted deoxystreptamine group, and tobramycin a typical aminoglycoside with 4,6-disubstituted deoxystreptamine (the deoxystreptamine moiety is shown in blue). Streptomycin does not contain a deoxystreptamine group and thus is an “atypical” aminoglycoside.
Tobramycin is clinically important in the treatment of infections due to Gram-negative bacteria and in particular Pseudomonas aeruginosa, against which it counts among the most effective antibiotics. Streptomycin is used in combination with other antibiotics in treating tuberculosis.
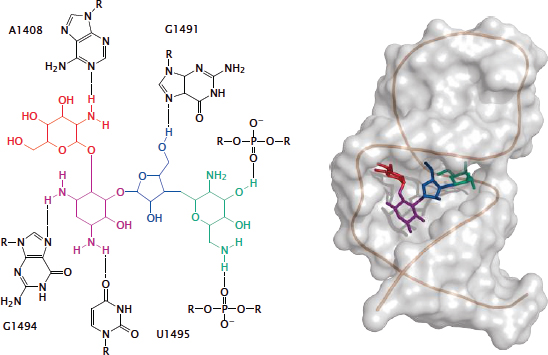
13.2.2 Paromomycin in the ribosomal aminoacyl acceptor site
Notes: Paromomycin is another aminoglycoside antibiotic. This three-dimensional structure of the RNA-paromomycin complex was determined by NMR spectroscopy, using a short model RNA that mimics the crucial double-stranded section of the (much larger) ribosomal RNA [100]. Rendered from 1pbr.pdb.
The “flattened” structure on the left shows the network of hydrogen bonds between the drug and the nucleotides of the ribosomal RNA. It can be seen that bonds involve both the bases and the backbone phosphates of the RNA.
13.2.3 Inactivation of kanamycin by resistance enzymes
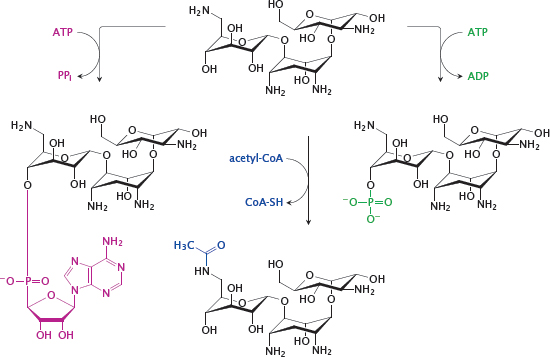
Notes: Aminoglycosides will be rendered inactive by covalent modification of the amino and hydroxyl groups that form hydrogen bonds with the bacterial RNA. Enzymes that perform such covalent modifications mediate bacterial resistance to these antibiotics.
This slide illustrates the reactions catalyzed by several resistance enzymes that inactivate kanamycin A, which is yet another aminoglycoside. Adenylylation (purple) is catalyzed by aminoglycoside O-adenylyltransferases, acetylation (blue) by amino-glycoside N-acetyltransferases, and phosphorylation (green) by aminoglycoside O-phosphotrans-ferases. The semisynthetic kanamycin derivative amikacin has been functionalized on an amino group that is not essential for binding to the ribosome. This modification protects the drug from some resistance enzymes. Amikacin is valuable in infections with Gram-negative bacteria that are difficult to treat otherwise.
13.2.4 Interactions of chloramphenicol with RNA in the peptidyl transferase site of the ribosome
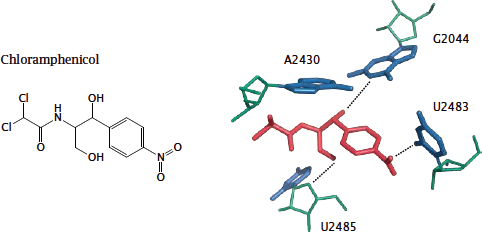
Notes: Chloramphenicol is a small, fairly lipophilic molecule with a broad spectrum of antibacterial activity and very favorable pharmacokinetic properties, but it is almost never used in developed countries because of allergic bone marrow toxicity.1 The drug binds within the peptidyltransferase site of the ribosome (see slide 11.5.1), which is part of the large subunit of the ribosome.
Like paromomycin, chloramphenicol interacts exclusively with the ribosomal RNA; this interaction involves both of its hydroxyl groups. The acetylation of one hydroxyl group by chloramphenicol acetyltransferase is an important resistance mechanism.
13.3 Novel RNA drug targets
The antibiotics discussed above show that ribosomal RNA makes a perfectly fine drug target, which suggests that RNA targets other than ribosomes should also be amenable to small drug molecules. This paradigm is still at the experimental stage.
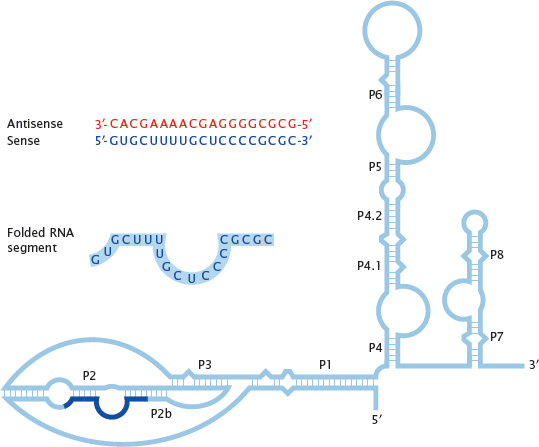
13.3.1 The RNA component of human telomerase
Notes: Telomerase is an enzyme that synthesizes repetitive DNA segments and attaches them to the telomeres, that is, the free ends of the chromosomes. Telomeres get shortened during each mitotic cell division, and if not renewed by telomerase, cumulative shortening during repeated cell divisions will ultimately wear them down entirely, putting an end to cell proliferation.