Chapter 9
Eicosanoids and related drugs
9.1 Overview
- “Local hormones”—sphere of action often limited to same anatomical site or tissue
- Involved in control of inflammation, fever, blood coagulation, pain perception, labor
- Derived from arachidonic acid and other polyunsaturated fatty acids, which occur in membrane phospholipids
- Most effects mediated by G protein-coupled receptors
- Some effects mediated by ion channels and nuclear hormone receptors
Notes: Eicosanoids are mediators that are derived from arachidonic acid and similar polyunsaturated fatty acids. The precursor fatty acids are stored as parts of the phospholipid molecules that make up the membranes of the endoplasmic reticulum and the nucleus. From these phospholipids, the fatty acids are mobilized on demand by phospholipase A2 and then enzymatically converted to the various mediators.
Among the many physiological functions that are regulated by eicosanoids, inflammation, pain perception and blood coagulation are of the greatest interest in pharmacotherapy.
9.1.1 Pathways and key enzymes in eicosanoid synthesis
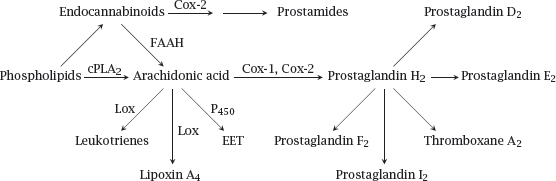
Notes: This slide introduces the major mediator classes and biosynthetic pathways. Release of arachidonic acid1 from membrane phospholipids by cytoplasmic phospholipase A2 (cPLA2) feeds cyclooxygenases 1 and 2 (Cox-1, Cox-2). These enzymes both form prostaglandin H2, which is then converted to other prostaglandins and to thromboxanes by specific synthases downstream.
Arachidonic acid is also the substrate of various lipoxygenases (Lox) and of some cytochrome P450 enzymes (P450), which produce leukotrienes, lipoxins, and epoxytrienoic acids (EET), respectively. Endocannabinoids, which are formed from arachidonatecontaining phospholipids through separate pathways, are cleaved to arachidonic acid by fatty acid amide hydrolase (FAAH). Alternatively, they may be metabolized first by Cox-2 and subsequently by one of the prostaglandin synthases, giving rise to prostamides.
9.1.2 Calcium signals activate cPLA2 and initiate the synthesis of prostaglandins and leukotrienes

Notes: The major storage lipid for arachidonic acid is phosphatidylinositol-bis-phosphate (PIP2). In the structure of PIP2, the arachidonyl residue is highlighted.
Synthesis of prostaglandins and leukotrienes is triggered by increased levels of cytosolic calcium, which may for example be induced by the phospholipase C pathway. Calcium allows cPLA2 to bind and attack the negatively charged PIP2. The arachidonic acid thus released is converted to eicosanoid precursors by membrane-associated cyclooxygenases (Cox) and lipoxygenases (Lox).
9.2 Biosynthetic pathways of prostaglandins and thromboxanes
Notes: Among the mediators synthesized downstream of cyclooxygenase and prostaglandin H2, thromboxane A2 triggers thrombocyte aggregation, while prostaglandins D-I are involved in pain perception, inflammation, and other physiological contexts.
Enzymes: Cyclooxygenase (1), thromboxane A synthase (2), prostaglandin D, E, F and I synthases (3–6).
9.2.1 Eicosanoids synthesized by lipoxygenases
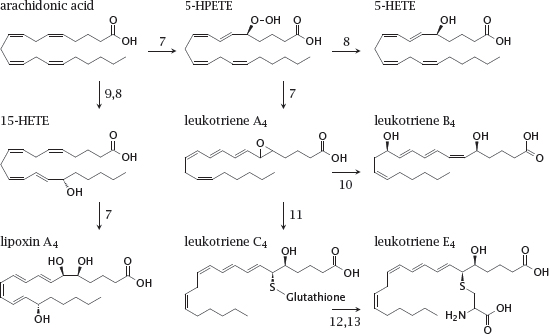
Enzymes: 5-lipoxygenase (7), HPETE peroxidase (8), 15-lipoxygenase (9), leukotriene A4 hydrolase (10), glutathione-S-transferase (11), peptidases (12,13).
Notes: Leukotrienes are proinflammatory mediators; they play a major role in the pathogenesis of asthma. Lipoxins are important in the resolution of inflammation, that is, they are antiinflammatory.
9.3 The two steps of the cyclooxygenase reaction
Notes: The cyclooxygenase reaction occurs in two stages, which are catalyzed in two separate active sites. In the cyclooxygenase site, arachidonic acid reacts with two molecules of O2 to acquire an endoperoxide and a hydroperoxide group; this yields the intermediate prostaglandin G2. The hydroperoxide is then reduced to a simple hydroxyl group in the peroxidase site, which gives prostaglandin H2.

While the two active sites are quite close in space, there is no direct pathway for the substrate between them. Prostaglandin G2 must therefore dissociate from the cyclooxygenase site and then rebind to the peroxidase site of the same or another enzyme molecule.
9.3.1 Reactions in the cyclooxygenase site

Notes: The key feature of the cyclooxygenase active site is a tyrosine residue (Tyr385 in Cox-1; highlighted in purple) that, in order to start the reaction, must be a radical. This radical abstracts a single hydrogen from arachidonic acid, which thereby itself turns into a radical. The radical electron then migrates and combines with an O2 π-diradical.
9.3.2 Reduction of prostaglandin G2 to prostaglandin H2
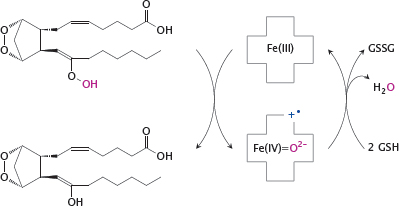
Notes: The peroxidase site contains heme as a prosthetic group. This heme “advances” one electron to each of the oxygens in the peroxy group of the substrate, reducing their formal charges from −1 to −2, and retains one oxygen atom. The heme itself is then reduced at the expense of two molecules of glutathione.
9.3.3 Interaction between the cyclooxygenase and peroxidase sites
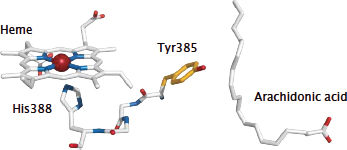
Notes: The two active sites in the cyclooxygenase molecule are close in space, and an interaction between them is required to convert the tyrosine in the cyclooxygenase site to its active radical form. In this interaction, an electron is abstracted from the reduced form of the tyrosine and consumed in the peroxidase reaction, replacing one of the electrons derived from glutathione in the regular peroxidase reaction.
In the picture, arachidonic acid is shown to indicate the location of the cyclooxygenase site; Tyr 385 is the catalytic tyrosine residue. The tyrosine is connected to the heme in the peroxidase site via a short stretch of just three amino acid residues, which reportedly act as the electron conduit between it and the heme.
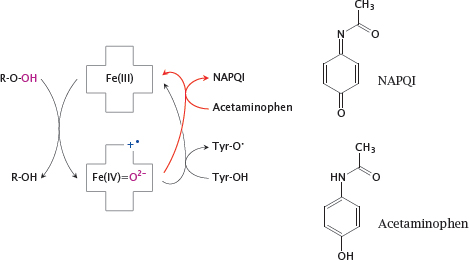
9.3.4 Priming of the tyrosine 385 radical, and mode of action of acetaminophen