Chapter 14
Drug delivery
14.1 Introduction
As we have seen in chapter 3, some drugs cannot get to their targets easily or even at all. In some cases, the best strategy is to use another drug altogether; however, in other cases, we can take measures to improve the uptake and retention of drugs, or to target them to specific sites of action in order to improve effectiveness and reduce toxicity. Such auxiliary techniques are collectively referred to as drug delivery. This chapter discusses a number of instructive examples.
14.2 Modifying drug molecules to improve intestinal uptake and distribution
We have already considered this topic earlier (see slide 3.4.5). Here, we will expand on it with several more examples of techniques and applications.
14.2.1 Protecting drugs from gastric acid through prodrug formation
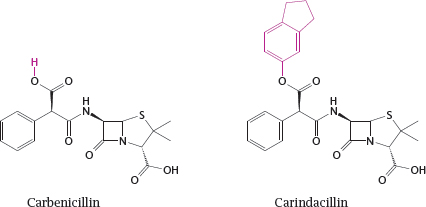
14.2.2 Optimizing a drug structure for bilayer permeation
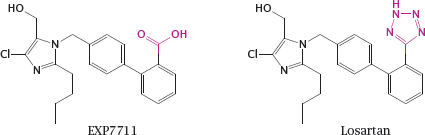
Notes: EXP7711 is an angiotensin receptor blocker with antihypertensive activity. The acidic carboxylate group in EXP7711 inhibits intestinal absorption, yet the negative charge in this position is necessary for tight receptor binding in order to achieve a high IC50.
Like carboxylate, tetrazole is acidic and has a planar structure, but at the same time it is ten times more lipophilic. Replacement of the carboxylate in EXP7711 with tetrazole gives losartan, which retains inhibitory activity on the angiotensin receptor (see slide 1.2.5) yet is absorbed from the intestine much more readily.
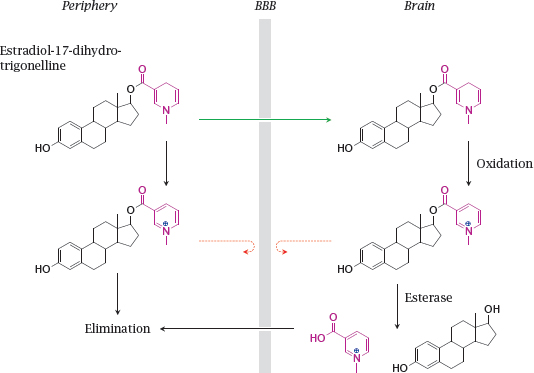
14.2.3 Trapping an estradiol prodrug inside the brain
14.2.4 Succinylsulfathiazole, a prodrug designed for reduced absorption
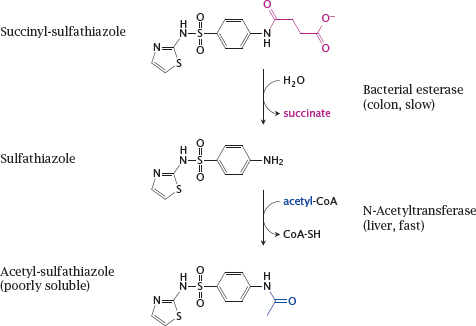
Notes: Sulfathiazole is a sulfonamide like sulfamidochrysoidine (see slide 1.3.3) and shares its mode of action. Sulfathiazole is metabolized through N-acetylation. The acetylated compound is poorly soluble, which may cause it to precipitate inside the kidney tubules; this may be lethal.
Succinylsulfathiazole is less toxic because it is ionized and thus not taken up efficiently. Once the drug reaches the large intestine, it is slowly hydrolyzed by bacterial esterases. The low rate of hydrolysis means that its uptake will also be slow and protracted; systemic concentrations of sulfathiazole and the acetylated metabolite will remain low throughout, and renal complications will be avoided.
While succinylsulfathiazole illustrates an interesting approach to mitigating drug toxicity, other sulfonamides that are not encumbered with this kind of potential problems in the first place are preferred in practice.
14.3 More on dopamine and its prodrugs
14.3.1 Inhibition of DOPA decarboxylase in the periphery improves L-DOPA uptake into the brain
Notes: As discussed earlier (slide 3.5.5), dopamine does not cross the blood brain barrier (BBB); therefore, in the treatment of Parkinson’s disease, its metabolic precursor L-DOPA is used as a prodrug. L-DOPA crosses the BBB by active transport and is converted to dopamine in the brain.
DOPA decarboxylase, the enzyme that converts L-DOPA to dopamine, is active both in the periphery and the CNS. Accordingly, if L-DOPA is applied alone, most of it undergoes decarboxylation in the periphery. The dopamine produced there cannot enter the brain but may instead be converted to norepinephrine or epinephrine and so cause side effects on blood pressure and glucose metabolism.
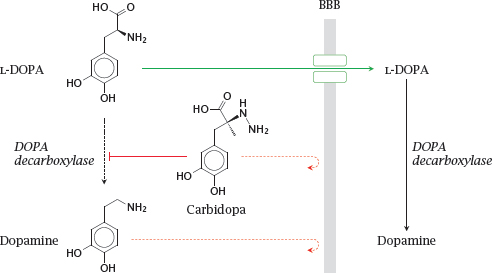
The premature conversion of L-DOPA can be prevented by combining L-DOPA with carbidopa or benserazide, which inhibit DOPA decarboxylase in the periphery. Like dopamine, these compounds do not cross the BBB and thus do not interfere with dopamine metabolism in the brain. Combination with such inhibitors permits the dosage of L-DOPA to be reduced by ~75% and very significantly reduces side effects.
14.3.2 Gludopa, a prodrug for selective release of dopamine in the kidneys
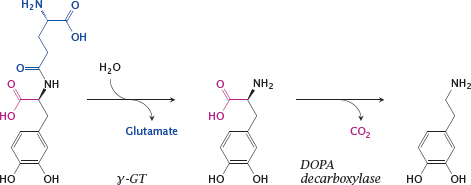
Notes: Dopamine receptors in the kidney vasculature improve kidney perfusion and function, and their selective stimulation may be clinically desirable. A prodrug to target dopamine preferentially to the kidneys is gludopa (γ-glutamyl-L-DOPA).
The kidneys (as well as the liver) contain L-γ-glutamyl-transpeptidase (γGT) at high levels of activity. This enzyme transfers γ-glutamyl residues between different substrates, but can also simply hydrolyze them. Hydrolytic removal of γ-glutamate from gludopa in the kidneys releases L-DOPA, which is again converted to dopamine and stimulates the local dopamine receptors.
14.4 Using carriers and vehicles to improve drug delivery
Aside from modifying the drug molecules themselves, the distribution or organ targeting of drugs can sometimes be improved by suitably packaging them before application.
14.4.1 Protecting drugs from gastric acid through acrylate copolymer coating
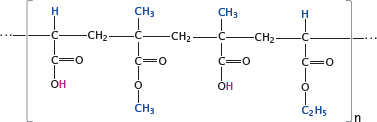
Notes: Some drug molecules contain acid-labile bonds such as carboxylic acid esters. These drugs must be protected from destruction by the fairly concentrated hydrochloric acid in the stomach. One way to achieve this protection is through micro-encapsulation in polymers that remain stable in an acidic milieu but dissolve once they reach the slightly alkaline environment within the small intestine. This technique is commonly referred to as enteric coating.
Widely used for this purpose are acrylate copolymers, often referred to using their trade name (Eudragit®). The acidic pH inside the stomach keeps the polymers’ carboxylic acid groups protonated, and the whole particles insoluble. Upon entering the small intestine, the carboxylic acid groups dissociate, and the ester bonds are cleaved. The polymers disperse and dissolve, releasing the cargo drugs enclosed within.
The slide shows one specific example, namely Eudragit® L 100-55; the substituents highlighted in blue differ in other commercial grades of this product (see [105] for details).
14.4.2 Site-selective delivery of BCNU
Notes: Bis-chloroethyl-nitrosurea is a bifunctional alkylating agent that is used in the treatment of cancer (see slide 12.5.18). The drug can cross the BBB in principle, but it is highly reactive and therefore consumed within a short range of penetration into the brain tissue, mostly before reaching the tumor. Therefore, systemic application for treating brain tumors is problematic.
For localized, sustained delivery in treating brain tumors, the drug is embedded in a polyanhydride polymer, and the product is shaped into wafers that go by the trade name Gliadel®. These are placed into the tumor resection cavity during tumor surgery.




