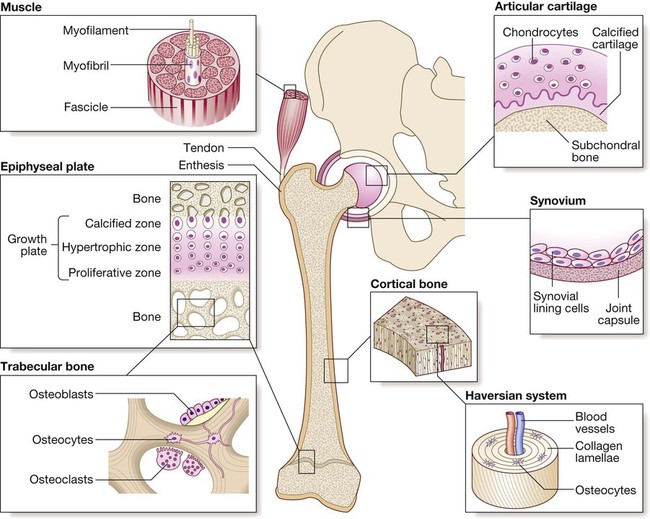
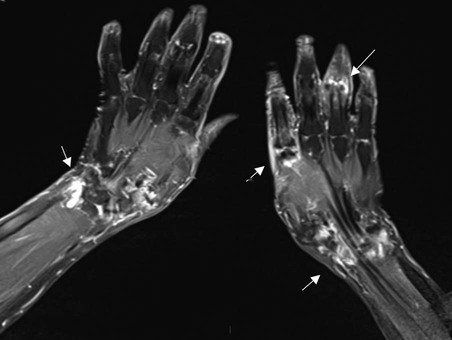
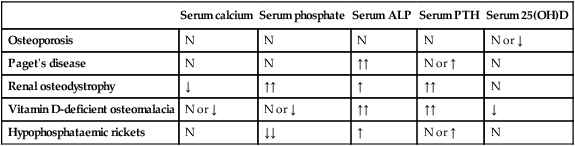
Diseases of the musculoskeletal system tend to be more common in women and most increase in frequency with increasing age. The two most common disorders are osteoarthritis and osteoporosis (Box 25.1). Osteoarthritis is the most common type of arthritis and affects up to 80% of people over the age of 75. Osteoporosis is the most common bone disease and affects 50% of women and 20% of men by their eighth decade. Diseases of the musculoskeletal system are the most common cause of physical disability in older people and account for one-third of physical disability at all ages. The musculoskeletal system is responsible for movement of the body, provides a structural framework to protect internal organs, and acts as a reservoir for storage of calcium and phosphate in the regulation of mineral homeostasis. The main components of the musculoskeletal system are depicted in Figure 25.1. The normal skeleton has two forms of bone tissue (see Fig. 25.1). Cortical bone is formed from Haversian systems, comprising concentric lamellae of bone tissue surrounding a central canal that contains blood vessels. Cortical bone is dense and forms a hard envelope around the long bones. Trabecular or cancellous bone fills the centre of the bone and consists of an interconnecting meshwork of trabeculae, separated by spaces filled with bone marrow. There are three main cell types in bone: • Osteoclasts: multinucleated cells of haematopoietic origin, responsible for bone resorption. • Osteoblasts: mononuclear cells of mesenchymal origin, responsible for bone formation. • Osteocytes: these differentiate from osteoblasts during bone formation and become embedded in bone matrix. Osteocytes are responsible for sensing and responding to mechanical loading of the skeleton and play a critical role in regulating bone formation and bone resorption, by producing receptor activator of nuclear factor kappa B ligand (RANKL) and sclerostin (SOST). They also play a central role in regulating phosphate metabolism by producing the hormone fibroblast growth factor 23 (FGF23), which acts on the kidney to promote phosphate excretion (Fig. 25.2). The most abundant protein of bone is type I collagen, which is formed from two α1 peptide chains and one α2 chain wound together in a triple helix. Type I collagen is proteolytically processed inside the cell before being laid down in the extracellular space, releasing propeptide fragments that can be used as biochemical markers of bone formation (p. 1066). Subsequently, the collagen fibrils become ‘cross-linked’ to one another by pyridinium molecules, a process that enhances bone strength. When bone is broken down by osteoclasts, the cross-links are released, providing biochemical markers of bone resorption. Bone is normally laid down in an orderly fashion, but when bone turnover is high, as in Paget’s disease or severe hyperparathyroidism, it is laid down in a chaotic pattern, giving rise to ‘woven bone’, which is mechanically weak. Bone matrix also contains growth factors, other structural proteins and proteoglycans, thought to be involved in helping bone cells attach to bone matrix and in regulating bone cell activity. The other major component of bone is mineral, comprised of calcium and phosphate crystals deposited between the collagen fibrils in the form of hydroxyapatite [Ca10 (PO4)6 (OH)2]. Mineralisation is essential for bone’s rigidity and strength, but over-mineralisation can increase brittleness, which contributes to bone fragility in diseases like osteogenesis imperfecta (p. 1131). Bone remodelling is required for renewal and repair of the skeleton throughout life (see Fig. 25.2). It starts with the attraction of osteoclast precursors in peripheral blood to the target site, probably by local release of chemotactic factors from areas of microdamage. The osteoclast precursors differentiate into mature osteoclasts in response to RANKL, which is produced by osteocytes, activated T cells and bone marrow stromal cells. RANKL activates the RANK receptor, which is expressed on osteoclasts and precursors. This is blocked by osteoprotegerin (OPG), a decoy receptor for RANKL that inhibits osteoclast formation. Mature osteoclasts attach to the bone surface by a tight sealing zone, and secrete hydrochloric acid and proteolytic enzymes such as cathepsin K into the space underneath. The acid dissolves the mineral and cathepsin K degrades collagen. When resorption is complete, osteoclasts undergo programmed cell death, and bone formation begins with the attraction of osteoblast precursors to the resorption site. These differentiate into mature osteoblasts, which deposit new bone matrix in the resorption lacuna, until the hole is filled. Some osteoblasts become trapped in bone matrix and differentiate into osteocytes. These act as biomechanical sensors and produce several molecules that influence bone remodelling and phosphate metabolism. Bone formation is stimulated by Wnt proteins, which bind to and activate lipoprotein-related receptor protein 5 (LRP5), expressed on osteoblasts. This process is inhibited by SOST, which is produced by osteocytes (see Fig. 25.2). Initially, the newly formed bone matrix (osteoid) is uncalcified but subsequently becomes mineralised to form mature bone. Alkaline phosphatase (ALP), produced by osteoblasts, plays an important role in bone mineralisation by degrading pyrophosphate, an inhibitor of mineralisation. Bone remodelling is regulated by circulating hormones such as parathyroid hormone (PTH) and oestrogen, and locally produced factors such as cytokines (Box 25.2). Many systemic hormones exert effects on bone turnover by affecting local expression of RANK, RANKL, OPG, SOST and molecules in the Wnt/LRP5 pathway (Fig. 25.2, Box 25.2). Bones are linked by joints. There are three main subtypes: fibrous, fibrocartilaginous and synovial (Box 25.3). Complex structures containing several cell types, these are found where a wide range of movement is needed (Fig. 25.3). This avascular tissue covers the bone ends in synovial joints. Cartilage cells (chondrocytes) are responsible for synthesis and turnover of cartilage, which consists of a mesh of type II collagen fibrils that extend through a hydrated ‘gel’ of proteoglycan molecules. The most important proteoglycan is aggrecan, which consists of a core protein to which several glycosaminoglycan (GAG) side-chains are attached (Fig. 25.4). The GAGs are polysaccharides that consist of long chains of disaccharide repeats comprising one normal sugar and an amino sugar. The most abundant GAGs in aggrecan are chondroitin sulphate and keratan sulphate. Hyaluronan is another important GAG that binds to aggrecan molecules to form very large complexes with a total molecular weight of more than 100 million. Aggrecan has a strong negative charge and avidly binds water molecules to assume a shape that occupies the maximum possible volume available. The expansive force of the hydrated aggrecan, combined with the restrictive strength of the collagen mesh, gives articular cartilage excellent shock-absorbing properties. With ageing, the concentration of chondroitin sulphate decreases, whereas that of keratan sulphate increases, resulting in reduced water content and shock-absorbing properties. These changes differ from those found in osteoarthritis (p. 1081), where there is abnormal chondrocyte division, loss of proteoglycan from matrix and an increase in water content. Cartilage matrix is constantly turning over and in health there is a perfect balance between synthesis and degradation. Degradation of cartilage matrix is carried out by aggrecanases and matrix metalloproteinases, responsible for the breakdown of proteins and proteoglycans, and by glycosidases, responsible for the breakdown of GAGs. Pro-inflammatory cytokines, such as interleukin-1 (IL-1) and tumour necrosis factor (TNF), stimulate production of aggrecanase and metalloproteinases, which contribute to cartilage degradation in inflammatory arthritis. The bones of synovial joints are connected by the joint capsule, a fibrous structure richly supplied with blood vessels, nerves and lymphatics, which encases the joint. Ligaments are discrete, regional thickenings of the capsule that act to stabilise joints (see Fig. 25.3). The inner surface of the joint capsule is the synovial membrane, comprising an outer layer of blood vessels and loose connective tissue that is rich in type I collagen, and an inner layer 1–4 cells thick consisting of two main cell types. Type A synoviocytes are phagocytic cells derived from the monocyte/macrophage lineage and are responsible for removing particulate matter from the joint cavity; type B synoviocytes are fibroblast-like cells that secrete synovial fluid. Most inflammatory and degenerative joint diseases associate with thickening of the synovial membrane and infiltration by lymphocytes, polymorphs and macrophages. Skeletal muscles are responsible for body movements and respiration. Muscle consists of bundles of cells (myocytes) embedded in fine connective tissue containing nerves and blood vessels. Myocytes are large, elongated, multinucleated cells formed by fusion of mononuclear precursors (myoblasts) in early embryonic life. The nuclei lie peripherally and the centre of the cell contains actin and myosin molecules, which interdigitate with one another to form the myofibrils that are responsible for muscle contraction. The molecular mechanisms of skeletal muscle contraction are the same as for cardiac muscle (p. 531). Myocytes contain many mitochondria that provide the large amounts of adenosine triphosphate (ATP) necessary for muscle contraction, and are rich in the protein myoglobin, which acts as a reservoir for oxygen during contraction. It is possible to obtain SF by aspiration from most peripheral joints, and only a small volume is required for diagnostic purposes. Normal SF is present in small volume, and is clear and either colourless or pale yellow with a high viscosity. It contains few cells. With joint inflammation, the volume increases, the cell count and the proportion of neutrophils rise (causing turbidity), and the viscosity reduces (due to enzymatic degradation of hyaluronan and aggrecan). Turbid fluid with a high neutrophil count occurs in sepsis, crystal arthritis and reactive arthritis. High concentrations of urate crystals or cholesterol can make SF appear white. Non-uniform blood-staining usually reflects needle trauma to the synovium. Uniform blood-staining is most commonly due to a bleeding diathesis, trauma or pigmented villonodular synovitis (p. 1134), but can occur in severe inflammatory synovitis. A lipid layer floating above blood-stained fluid is diagnostic of intra-articular fracture and is caused by release of bone marrow fat into the joint. Crystals can be identified by compensated polarised light microscopy of fresh SF (to avoid crystal dissolution and post-aspiration crystallisation). Urate crystals are long and needle-shaped, and show a strong light intensity and negative birefringence (Fig. 25.5A). Calcium pyrophosphate crystals are smaller, rhomboid in shape and usually less numerous than urate, and have weak intensity and positive birefringence (Fig. 25.5B). Radiographs show anatomical changes that are of value in the differential diagnosis of many bone and joint diseases (Box 25.4). The bones and joints to be X-rayed are usually selected on the basis of symptoms or patterns of involvement identified at clinical assessment. Radiographs are of diagnostic value in osteoarthritis (OA), where they demonstrate joint space narrowing that tends to be focal rather than widespread, as in inflammatory arthritis. Other features of OA detected on X-ray include osteophytes, subchondral sclerosis, bone cysts and calcified loose bodies within the synovium (see Fig. 25.20, p. 1084). Radiographs may show erosions and sclerosis of the sacroiliac joints and syndesmophytes in the spine in patients with seronegative spondyloarthritis (see Fig. 25.36, p. 1106). In peripheral joints, so-called proliferative erosions, associated with new bone formation and a periosteal reaction, may be observed. In tophaceous gout, well-defined punched-out erosions may occur (see Fig. 25.26, p. 1089). Calcification of cartilage, tendons and soft tissues or muscle may occur in chondrocalcinosis (see Fig. 25.27, p. 1090), calcific periarthritis and connective tissue diseases. This is useful in patients suspected of having metastatic bone disease and Paget’s disease. It involves gamma-camera imaging following an intravenous injection of 99mTc-bisphosphonate. Early post-injection images reflect vascularity and can show increased perfusion of inflamed synovium, Pagetic bone, or primary or secondary bone tumours. Delayed images taken a few hours later reflect bone remodelling as the bisphosphonate localises to sites of active bone turnover. Scintigraphy has a high sensitivity for detecting important bone and joint pathology that is not apparent on plain X-rays (Box 25.5). Single photon emission computed tomography (SPECT) combines radionuclide imaging with computed tomography. It can provide accurate anatomical localisation of abnormal tracer uptake within the bone and is of particular value in the assessment of patients with chronic low back pain of unknown cause. Magnetic resonance imaging (MRI) gives detailed information on anatomy, allowing three-dimensional visualisation of bone and soft tissues that cannot be adequately assessed by plain X-rays. The technique is valuable in the assessment and diagnosis of many musculoskeletal diseases (Box 25.6). T1-weighted sequences are useful for defining anatomy, whereas T2-weighted sequences are useful for assessing tissue water content, which is often increased in synovitis and other inflammatory disorders (Fig. 25.6). Contrast agents, such as gadolinium, can be administered to increase sensitivity in detecting erosions and synovitis. Ultrasonography is a useful investigation for confirmation of small joint synovitis and erosion, for anatomical confirmation of periarticular lesions, and for guided aspiration and injection of joints and bursae. Ultrasound is more sensitive than clinical examination for the detection of early synovitis and is used increasingly in the diagnosis and assessment of patients with suspected inflammatory arthritis. In addition to locating synovial thickening and effusions, ultrasound can detect increased blood flow within synovium using power Doppler imaging, an option that is available on most modern ultrasound machines (Fig. 25.7). Bone mineral density (BMD) measurements play a key role in the diagnosis and management of osteoporosis. The technique of choice is dual energy X-ray absorptiometry (DEXA), which is usually performed at the lumbar spine and hip, and provides images of the region studied. This technique works on the principle that calcium in bone attenuates passage of X-ray beams through the tissue in proportion to the amount of mineral present. The greater the amount of bone mineral present, the higher the BMD value. Most DEXA scanners give a BMD readout expressed as grams of hydroxyapatite/cm2, and as a T-score and Z-score value. The T-score is a measure of the number of standard deviations by which the patient’s BMD value differs from that in a young healthy control, whereas the BMD Z-score is a measure of the number of standard deviations by which the BMD deviates from that of age-matched controls (Fig. 25.8). Osteoporosis is defined by a T-score value of 2.5 or below (shaded red in the figure), whereas osteopenia is diagnosed when the T-score lies between −1.0 and −2.5 (shaded pink). Many healthy people, especially above the age of 50, have BMD values in the osteopenic range. Values of BMD above −1.0 and below +2.5 are considered normal, whereas values above +2.5 can be found in osteosclerotic diseases and OA. Results need to be interpreted carefully. It is possible for BMD values to lie in the normal or osteopenic range in patients who have osteoporosis, due to coexisting conditions such as aortic calcification, vertebral compression fractures, degenerative disc disease and OA. These can sometimes be suspected on the basis of antero-posterior or lateral DEXA images, but abnormal appearances should be confirmed by X-ray or other imaging as appropriate. Routine biochemistry is useful for assessing metabolic bone disease, muscle diseases and gout. Several bone diseases, including Paget’s disease, renal bone disease and osteomalacia, give a characteristic pattern that can be helpful diagnostically (Box 25.7). Serum levels of uric acid are usually raised in gout but a normal level does not exclude it, especially during an acute attack, when urate levels temporarily fall. Equally, an elevated serum uric acid does not confirm the diagnosis, since most hyperuricaemic people never develop gout. Levels of C-reactive protein (CRP) are a useful marker of infection and inflammation, and are more specific than the ESR. An exception is in connective tissue diseases such as systemic lupus erythematosus (SLE) and systemic sclerosis, where CRP may be normal but ESR raised in active inflammatory disease. Accordingly, an elevated CRP in a patient with lupus or scleroderma suggests an intercurrent illness such as sepsis rather than active disease. More detail on the interpretation of CRP and ESR changes is given on page 84. Serum creatine kinase (CK) levels are useful in the diagnosis of myopathy or myositis, but specificity and sensitivity are poor and raised levels may occur in some conditions (Box 25.8). Biochemical monitoring of renal and hepatic function is important in ongoing care of patients on DMARD therapy.
Rheumatology and bone disease
Clinical examination of the musculoskeletal system
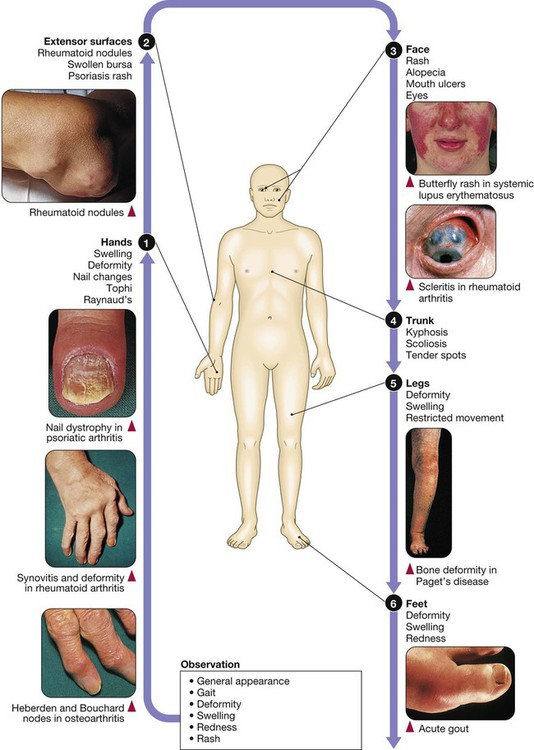
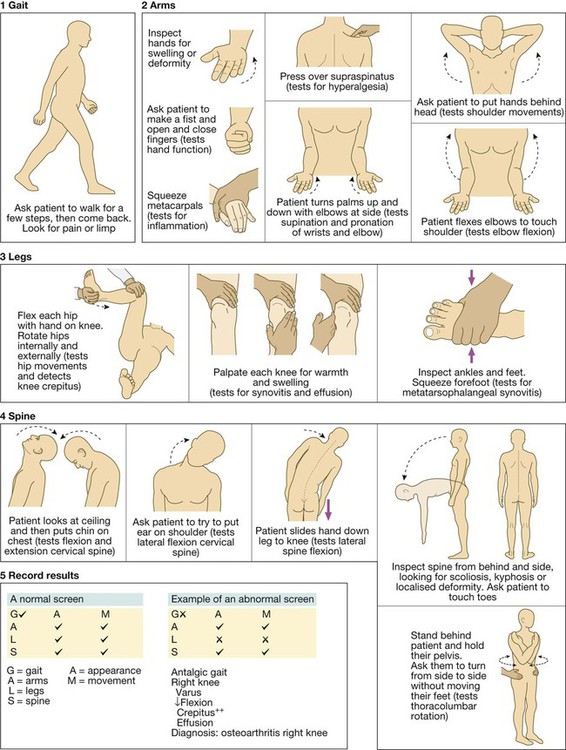
General Assessment of Locomotor System (GALS)
Functional anatomy and physiology
Bone
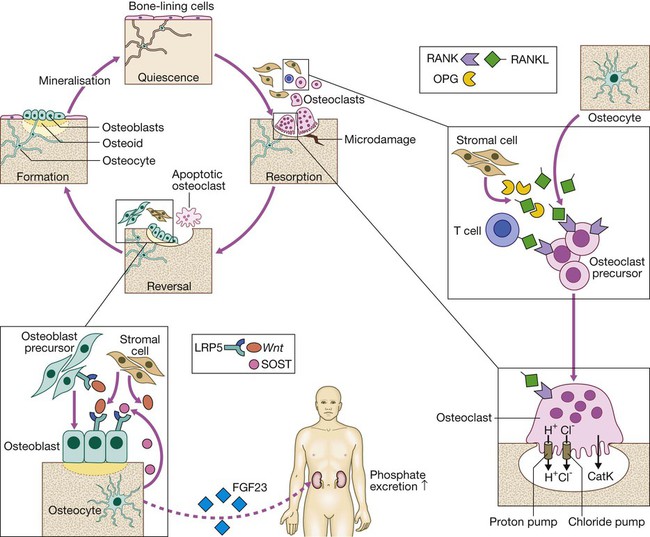
Bone is renewed and repaired during the bone remodelling cycle, in which old and damaged bone is removed by osteoclasts and replaced by osteoblasts. Osteocytes play a central role in bone remodelling by secreting RANKL, which promotes osteoclast differentiation and activity by binding to RANK. Osteocytes regulate bone formation by producing SOST, which binds to the LRP5 receptor and prevents its activation by members of the Wnt family. Osteocytes also regulate phosphate homeostasis by producing fibroblast growth factor 23 (FGF23), which is a circulating hormone that acts on the kidney to promote phosphate excretion. (CatK = cathepsin K; LRP5 = lipoprotein receptor protein 5; OPG = osteoprotegerin; RANK = receptor activator of nuclear factor kappa B; RANKL = RANK ligand; SOST = sclerostin)
Bone matrix and mineral
Bone remodelling
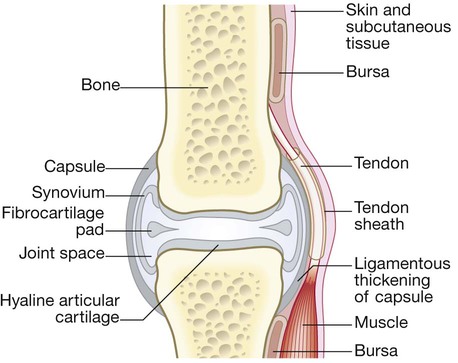
Joints
Synovial joints
Articular cartilage
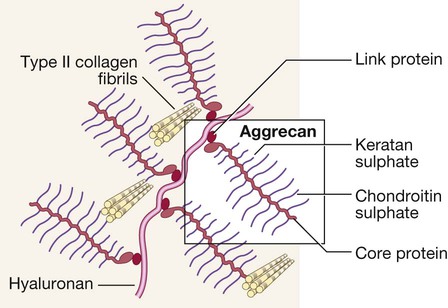
The synovial membrane, joint capsule and bursae
Skeletal muscle
Investigation of musculoskeletal disease
Joint aspiration
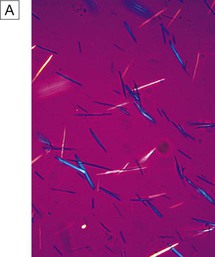
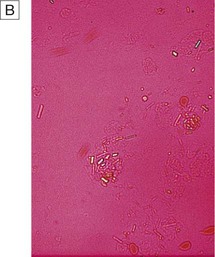
A Monosodium urate crystals showing bright negative birefringence under polarised light and needle-shaped morphology. B Calcium pyrophosphate crystals showing weak positive birefringence under polarised light and are few in number. They are more difficult to detect than urate crystals.
Imaging
Plain radiography
Radionuclide bone scan
Magnetic resonance imaging
Ultrasonography
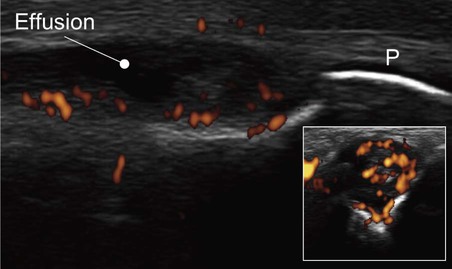
Lateral image of a metacarpophalangeal joint in inflammatory arthritis. The periosteum (P) of the phalanx shows as a white line. The dark, hypo-echoic area indicates an effusion. The coloured areas demonstrated by power Doppler indicate increased vascularity. The inset shows a transverse image of the same joint.
Bone mineral density
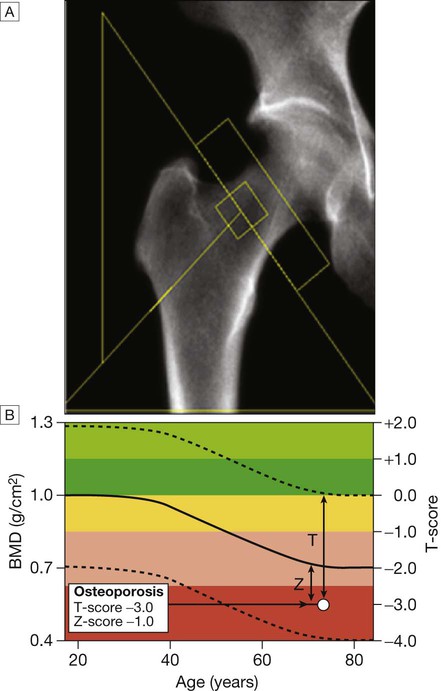
A DEXA scan of the hip. B Bone mineral density (BMD) values plotted in g/cm2 (left axis) and as the T-score values (right axis). The solid line represents the population average plotted against age, and the interrupted lines are ± 2 standard deviations from the average. The patient shown, aged 72, has an osteoporotic T-score of −3.0 but a Z-score of −1.0, which is within the ‘normal range’ for that age, reflecting the fact that bone is lost with age.
Blood tests
Haematology
Biochemistry