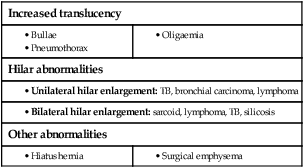
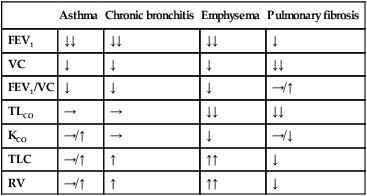
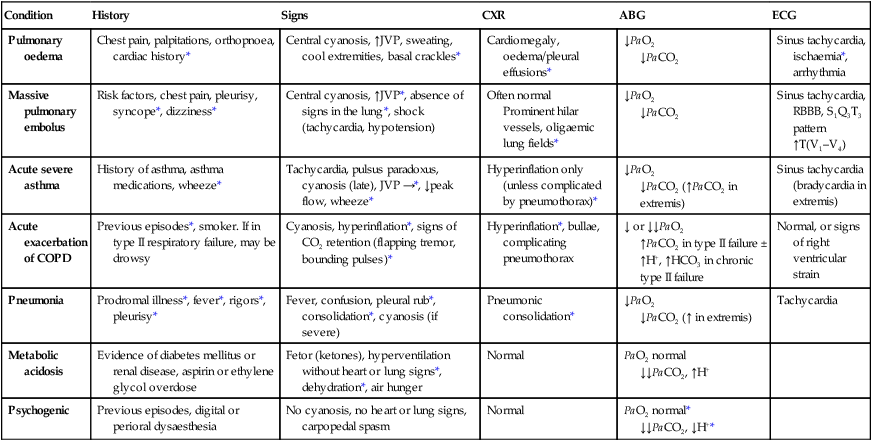
Respiratory disease is responsible for a major burden of morbidity and untimely death, with conditions such as tuberculosis, pandemic influenza and pneumonia the most important in world health terms. The increasing prevalence of allergy, asthma and chronic obstructive pulmonary disease (COPD) contributes to the overall burden of chronic disease in the community. By 2025, the number of cigarette smokers worldwide is anticipated to increase to 1.5 billion, ensuring a growing burden of tobacco-related respiratory conditions. The multitude of small airways within the lung parenchyma has a very large combined cross-sectional area (over 300 cm2 in the third-generation respiratory bronchioles), resulting in very slow flow rates. Airflow is normally silent here, and gas transport occurs largely by diffusion in the final generations. Major bronchial and pulmonary divisions are shown in Figure 19.1. The acinus (Fig. 19.2) is the gas exchange unit of the lung and comprises branching respiratory bronchioles and clusters of alveoli. Here the air makes close contact with the blood in the pulmonary capillaries (gas-to-blood distance < 0.4 µm), and oxygen uptake and CO2 excretion occur. The alveoli are lined with flattened epithelial cells (type I pneumocytes) and a few, more cuboidal, type II pneumocytes. The latter produce surfactant, which is a mixture of phospholipids that reduces surface tension and counteracts the tendency of alveoli to collapse under surface tension. Type II pneumocytes can divide to reconstitute type I pneumocytes after lung injury. Healthy alveolar walls contain a fine network of elastin and collagen fibres (see Fig. 19.2). The volume of the lungs at the end of a tidal (‘normal’) breath out is called the functional residual capacity (FRC). At this volume, the inward elastic recoil of the lungs (resulting from elastin fibres and surface tension in the alveolar lining fluid) is balanced by the resistance of the chest wall to inward distortion from its resting shape, causing negative pressure in the pleural space. Elastin fibres allow the lung to be easily distended at physiological lung volumes, but collagen fibres cause increasing stiffness as full inflation is approached so that, in health, the maximum inspiratory volume is limited by the lung (rather than the chest wall). Within the lung, the weight of tissue compresses the dependent regions and distends the uppermost parts, so a greater portion of an inhaled breath passes to the basal regions, which also receive the greatest blood flow as a result of gravity. Elastin fibres in alveolar walls maintain small airway patency by radial traction on the airway walls. Even in health, however, these small airways narrow during expiration because they are surrounded by alveoli at higher pressure, but are prevented from collapsing by radial elastic traction. The volume that can be exhaled is thus limited purely by the capacity of the expiratory muscles to distort the chest wall inwards. In emphysema, loss of alveolar walls leaves the small airways unsupported, and their collapse on expiration causes air trapping and high end-expiratory volume (p. 673). The respiratory motor neurons in the posterior medulla oblongata are the origin of the respiratory cycle. Their activity is modulated by multiple external inputs in health and in disease (see Fig. 19.10, p. 657): • Central chemoreceptors in the ventrolateral medulla sense the pH of the cerebrospinal fluid (CSF) and are indirectly stimulated by a rise in arterial PCO2. • The carotid bodies sense hypoxaemia but are mainly activated by arterial PO2 values below 8 KPa (60 mmHg). They are also sensitised to hypoxia by raised arterial PCO2. • Muscle spindles in the respiratory muscles sense changes in mechanical load. • Vagal sensory fibres from the lung may be stimulated by stretch, by inhaled toxins or by disease processes in the interstitium. • Cortical (volitional) and limbic (emotional) influences can override the automatic control of breathing. To achieve optimal gas exchange within the lungs, the regional distribution of ventilation and perfusion must be matched. At segmental and subsegmental level, hypoxia constricts pulmonary arterioles and airway CO2 dilates bronchi, helping to maintain good regional matching of ventilation and perfusion. Lung disease may create regions of relative underventilation or underperfusion, which disturb this regional matching, causing respiratory failure (p. 663). In addition to causing ventilation–perfusion mismatch, diseases that destroy capillaries or thicken the alveolar capillary membrane (e.g. emphysema or fibrosis) can impair gas diffusion directly. Large airborne particles are trapped by nasal hairs, and smaller particles settling on the mucosa are cleared towards the oropharynx by the columnar ciliated epithelium which covers the turbinates and septum (Fig. 19.3). During cough, expiratory muscle effort against a closed glottis results in high intrathoracic pressure, which is then released explosively. The flexible posterior tracheal wall is pushed inwards by the high surrounding pressure, which reduces tracheal cross-section and thus maximises the airspeed to achieve effective expectoration. The larynx also acts as a sphincter, protecting the airway during swallowing and vomiting. This is performed on the majority of patients suspected of having chest disease. A postero-anterior (PA) film provides information on the lung fields, heart, mediastinum, vascular structures and thoracic cage (Fig. 19.4). Additional information may be obtained from a lateral film, particularly if pathology is suspected behind the heart shadow or deep in the diaphragmatic sulci. An approach to interpreting the chest X-ray is given in Box 19.2, and common abnormalities are listed in Box 19.3. Increased shadowing may represent accumulation of fluid, lobar collapse or consolidation. Uncomplicated consolidation should not change the position of the mediastinum and the presence of an air bronchogram means that proximal bronchi are patent. Collapse (implying obstruction of the lobar bronchus) is accompanied by loss of volume and displacement of the mediastinum towards the affected side (Fig. 19.5). The presence of ring shadows (thickened bronchi seen end-on), tramline shadows (thickened bronchi side-on) or tubular shadows (bronchi filled with secretions) suggests bronchiectasis, but computed tomography is a much more sensitive test than plain X-ray in bronchiectasis. The presence of pleural fluid is suggested by a dense basal shadow, which, in the erect patient, ascends towards the axilla (p. 645). In large pulmonary embolism, relative oligaemia may cause a lung field to appear abnormally dark. High-resolution CT (HRCT) uses thin sections to provide detailed images of the pulmonary parenchyma and is particularly useful in assessing diffuse parenchymal lung disease, identifying bronchiectasis (Fig. 19.30, p. 679), and assessing type and extent of emphysema. CT pulmonary angiography (CTPA) has become the investigation of choice in the diagnosis of pulmonary thromboembolism (see Fig. 19.69, p. 679), when it may either confirm the suspected embolism or highlight an alternative diagnosis. It has largely replaced the radioisotope-based ventilation–perfusion scan, although the latter continues to provide useful information in the pre-operative assessment of patients being considered for lung resection. In pulmonary hypertension, Doppler echocardiographic assessment of tricuspid regurgitant jets allows accurate non-invasive measurement of pulmonary artery pressure in most cases. Right heart catheterisation is still used in the investigation of patients with pulmonary hypertension in specialised centres, as it permits accurate measurement of response to pulmonary vasodilators. Positron emission tomography (PET) scanners exploit the ability of malignant tissue to absorb and metabolise glucose avidly. The radiotracer 18F-fluorodeoxyglucose (FDG) is infused and rapidly taken up by malignant tissue. It is then phosphorylated but cannot be metabolised further, becoming ‘trapped’ in the cell. PET is useful in the investigation of pulmonary nodules, and in staging mediastinal lymph nodes and distal metastatic disease in patients with lung cancer. The negative predictive value is high; however, the positive predictive value is poor. Co-registration of PET and CT (PET-CT) enhances localisation and characterisation of metabolically active deposits (Fig. 19.6). Future advances will see the combination of PET and magnetic resonance imaging (MRI). The trachea and the first 3–4 generations of bronchi may be inspected using a flexible bronchoscope. Flexible bronchoscopy is usually performed under local anaesthesia with sedation, on an outpatient basis. Abnormal tissue in the bronchial lumen or wall can be biopsied, and bronchial brushings, washings or aspirates can be taken for cytological or bacteriological examination. Small biopsy specimens of lung tissue, taken by forceps passed through the bronchial wall (transbronchial biopsies), may be helpful in the diagnosis of bronchocentric disorders such as sarcoid, hypersensitivity pneumonitis and diffuse malignancy, but are generally too small to be of diagnostic value in other diffuse parenchymal pulmonary disease (p. 706). Transbronchial needle aspiration (TBNA) may be used to sample mediastinal lymph nodes and to stage lung cancer. Rigid bronchoscopy requires general anaesthesia and is reserved for specific situations, such as massive haemoptysis or removal of foreign bodies (see Fig. 19.9, p. 655). Endobronchial laser therapy and endobronchial stenting may be easier with rigid bronchoscopy. The presence of pneumococcal antigen (revealed by counter-immunoelectrophoresis) in sputum, blood or urine may be of diagnostic importance in pneumonia. Influenza viruses can be detected in throat swab samples by fluorescent antibody techniques. In blood, high or rising antibody titres to specific organisms (such as Legionella, Mycoplasma, Chlamydia or viruses) may eventually clinch a diagnosis suspected on clinical grounds but early diagnosis of Legionella is best done by urine antigen testing. Precipitating antibodies may indicate a reaction to fungi such as Aspergillus (p. 697) or to antigens involved in hypersensitivity pneumonitis (p. 719). Total levels of immunoglobulin E (IgE), and levels of IgE directed against specific antigens, can be useful in assessing the contribution of allergy to respiratory disease. Cytological examination of exfoliated cells in pleural fluid or bronchial brushings and washings, or of fine needle aspirates from lymph nodes or pulmonary lesions, can support a diagnosis of malignancy but, if this is indeterminate, a larger tissue biopsy is often necessary. Differential cell counts in bronchial lavage fluid may help to distinguish pulmonary changes due to sarcoidosis (p. 709) from those caused by idiopathic pulmonary fibrosis (p. 706) or hypersensitivity pneumonitis (p. 719). Respiratory function tests are used to aid diagnosis, assess functional impairment, and monitor treatment or progression of disease. Airway narrowing, lung volume and gas exchange capacity are quantified and compared with normal values adjusted for age, gender, height and ethnic origin. In diseases characterised by airway narrowing (e.g. asthma, bronchitis and emphysema), maximum expiratory flow is limited by dynamic compression of small intrathoracic airways, some of which may close completely during expiration, limiting the volume that can be expired (‘obstructive’ defect). Hyperinflation of the chest results, and can become extreme if elastic recoil is also lost due to parenchymal destruction, as in emphysema. In contrast, diseases that cause interstitial inflammation and/or fibrosis lead to progressive loss of lung volume (‘restrictive’ defect) with normal expiratory flow rates. Typical laboratory traces are illustrated in Figure 19.7. Airway narrowing is assessed by asking patients to blow out as hard and as fast as they can into a peak flow meter or a spirometer. Peak flow meters are cheap and convenient for home monitoring of peak expiratory flow (PEF) in the detection and monitoring of asthma, but results are effort-dependent. More accurate and reproducible measures are obtained by inhaling fully, then exhaling at maximum effort into a spirometer. The forced expired volume in 1 second (FEV1) is the volume exhaled in the first second, and the forced vital capacity (FVC) is the total volume exhaled. FEV1 is disproportionately reduced in airflow obstruction, resulting in FEV1/FVC ratios of less than 70%. In this situation, spirometry should be repeated following inhaled short-acting β2-adrenoceptor agonists (e.g. salbutamol); a large improvement in FEV1 (over 400 mL) and variability in peak flow over time are features of asthma (p. 668). To distinguish large airway narrowing (e.g. tracheal stenosis or compression) from small airway narrowing, flow/volume loops are recorded using spirometry. These display flow in relation to lung volume (rather than time) during maximum expiration and inspiration, and the pattern of flow reveals the site of airflow obstruction (see Fig. 19.7). To measure the capacity of the lungs to exchange gas, patients inhale a test mixture of 0.3% carbon monoxide, which is taken up avidly by haemoglobin in pulmonary capillaries. After a short breath-hold, the rate of disappearance of CO into the circulation is calculated from a sample of expirate, and expressed as the TLCO or carbon monoxide transfer factor. Helium is also included in the test breath to allow calculation of the volume of lung examined by the test breath. Transfer factor expressed per unit lung volume is termed KCO. Common respiratory function abnormalities are summarised in Box 19.4. The measurement of hydrogen ion concentration, PaO2 and PaCO2, and derived bicarbonate concentration in an arterial blood sample is essential to assess the degree and type of respiratory failure, and for measuring acid–base status. This is discussed in detail on pages 663 and 442. Interpretation of results is made easier by blood gas diagrams (Fig. 19.8), which indicate whether any acidosis or alkalosis is due to acute or chronic respiratory derangements of PaCO2, or to metabolic causes. Pulse oximeters with finger or ear probes measure the difference in absorbance of light by oxygenated and deoxygenated blood to calculate its oxygen saturation (SaO2). This allows non-invasive continuous assessment of oxygen saturation in patients, which is useful in assessing hypoxaemia and its response to therapy. Cough is the most frequent symptom of respiratory disease and is caused by stimulation of sensory nerves in the mucosa of the pharynx, larynx, trachea and bronchi. Acute sensitisation of the normal cough reflex occurs in a number of conditions, and it is typically induced by changes in air temperature or exposure to irritants, such as cigarette smoke or perfumes. The characteristics of cough originating at various levels of the respiratory tract are detailed in Box 19.5. The explosive quality of a normal cough is lost in patients with respiratory muscle paralysis or vocal cord palsy. Paralysis of a single vocal cord gives rise to a prolonged, low-pitched, inefficient ‘bovine’ cough accompanied by hoarseness. Coexistence of an inspiratory noise (stridor) indicates partial obstruction of a major airway (e.g. laryngeal oedema, tracheal tumour, scarring, compression or inhaled foreign body) and requires urgent investigation and treatment. Sputum production is common in patients with acute or chronic cough, and its nature and appearance can provide clues to the aetiology (p. 644). Patients with chronic cough present more of a challenge, especially when physical examination, chest X-ray and lung function studies are normal. In this context, it is most often explained by cough-variant asthma (where cough may be the principal or exclusive clinical manifestation), post-nasal drip secondary to nasal or sinus disease, or gastro-oesophageal reflux with aspiration. Diagnosis of the latter may require ambulatory oesophageal pH monitoring or a prolonged trial of anti-reflux therapy (p. 865). Between 10% and 15% of patients (particularly women) taking angiotensin-converting enzyme (ACE) inhibitors develop a drug-induced chronic cough. Bordetella pertussis infection in adults (p. 682) can also result in protracted cough and should be suspected in those in close contact with children. While most patients with a bronchogenic carcinoma have an abnormal chest X-ray on presentation, fibreoptic bronchoscopy or thoracic CT is advisable in most adults (especially smokers) with otherwise unexplained cough of recent onset, as this may reveal a small endobronchial tumour or unexpected foreign body (Fig. 19.9). In a small percentage of patients, dry cough may be the presenting feature of interstitial lung disease. Stimuli to breathing resulting from disease processes are shown in Figure 19.10. Respiratory diseases can stimulate breathing and dyspnoea by: • stimulating intrapulmonary sensory nerves (e.g. pneumothorax, interstitial inflammation and pulmonary embolus) • increasing the mechanical load on the respiratory muscles (e.g. airflow obstruction or pulmonary fibrosis) • causing hypoxia, hypercapnia or acidosis, which stimulate chemoreceptors. Digital or perioral paraesthesiae and a feeling that ‘I cannot get a deep enough breath in’ are typical features of psychogenic hyperventilation, but this cannot be diagnosed until investigations have excluded other potential causes. Additional symptoms include lightheadedness, central chest discomfort or even carpopedal spasm due to acute respiratory alkalosis. These alarming symptoms may provoke further anxiety and exacerbate hyperventilation. Psychogenic breathlessness rarely disturbs sleep, frequently occurs at rest, may be provoked by stressful situations and may even be relieved by exercise. The Nijmegen questionnaire can be used to score some of the typical symptoms of hyperventilation (Box 19.7). Arterial blood gases show normal PO2, low PCO2 and alkalosis. This is one of the most common and dramatic medical emergencies. The history and a rapid but careful examination will usually suggest a diagnosis which can be confirmed by routine investigations, including chest X-ray, ECG and arterial blood gases. Specific features that aid the diagnosis of the important causes are shown in Box 19.8. It is important to establish the rate of onset and severity of the breathlessness and whether associated cardiovascular symptoms (chest pain, palpitations, sweating and nausea) or respiratory symptoms (cough, wheeze, haemoptysis, stridor – Fig. 19.11) are present. A previous history of repeated episodes of left ventricular failure, asthma or exacerbations of COPD is valuable. In the severely ill patient, it may be necessary to obtain the history from accompanying witnesses. In children, the possibility of inhalation of a foreign body (see Fig. 19.9) or acute epiglottitis should always be considered.
Respiratory disease
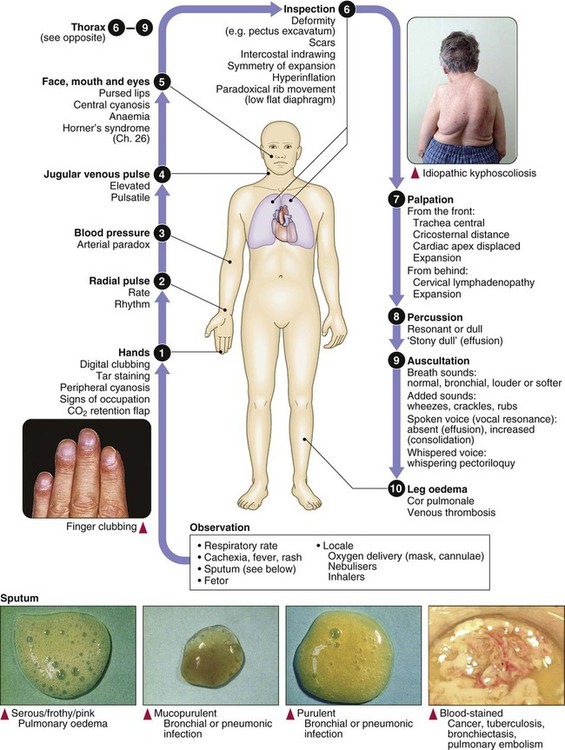
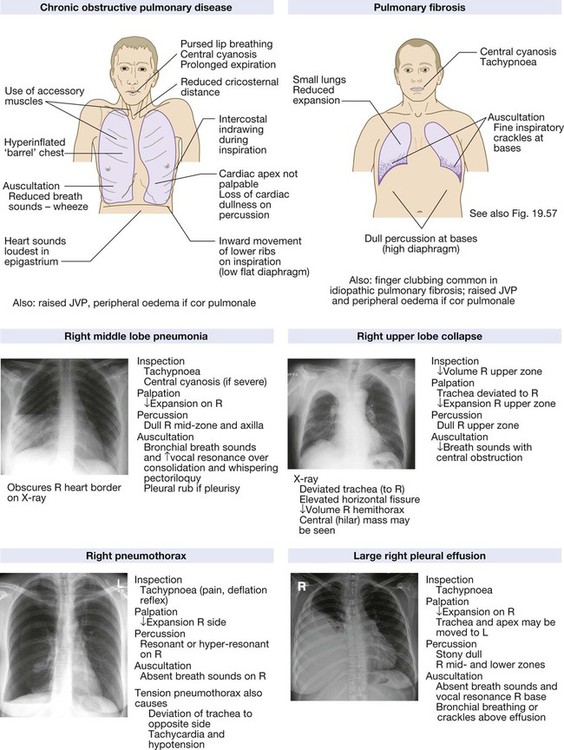
Clinical examination of the respiratory system
Functional anatomy and physiology
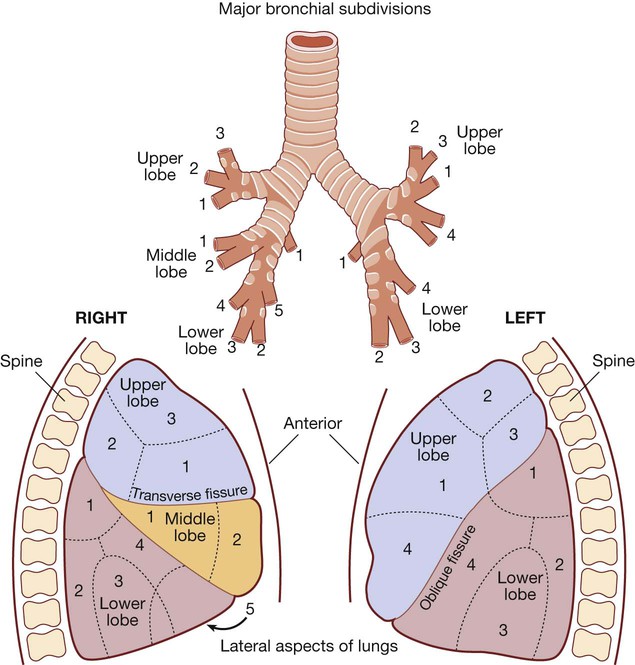
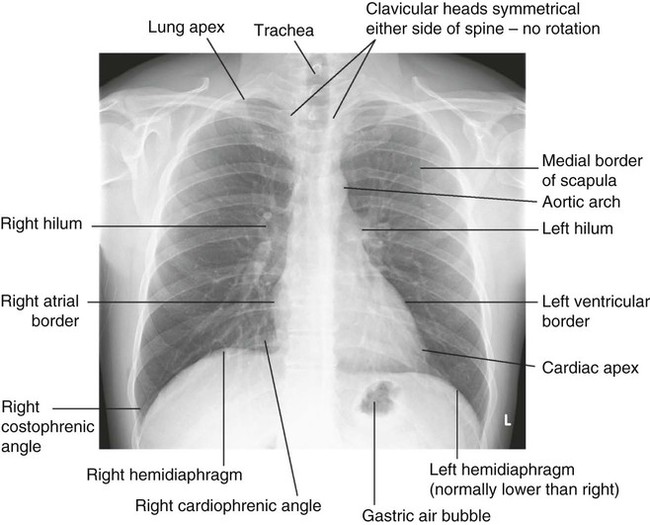
The angle of the oblique fissure means that the left upper lobe is largely anterior to the lower lobe. On the right, the transverse fissure separates the upper from the anteriorly placed middle lobe, which is matched by the lingular segment on the left side. The site of a lobe determines whether physical signs are mainly anterior or posterior. Each lobe is composed of two or more bronchopulmonary segments that are supplied by the main branches of each lobar bronchus.
Bronchopulmonary segments:
Right Upper lobe: (1) Anterior, (2) Posterior, (3) Apical. Middle lobe: (1) Lateral, (2) Medial. Lower lobe: (1) Apical, (2) Posterior basal, (3) Lateral basal, (4) Anterior basal, (5) Medial basal.
Left Upper lobe: (1) Anterior, (2) Apical, (3) Posterior, (4) Lingular. Lower lobe: (1) Apical, (2) Posterior basal, (3) Lateral basal, (4) Anterior basal.
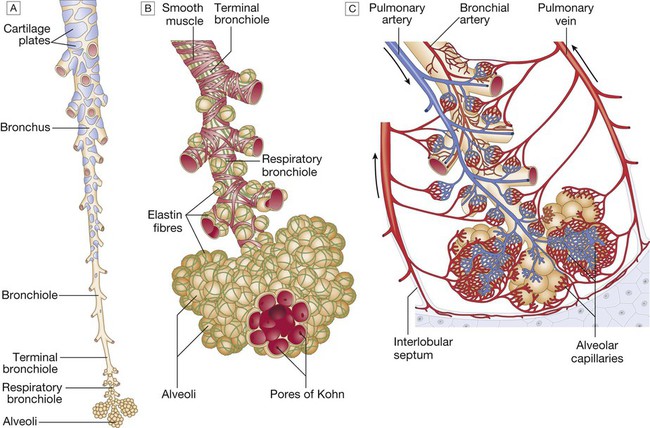
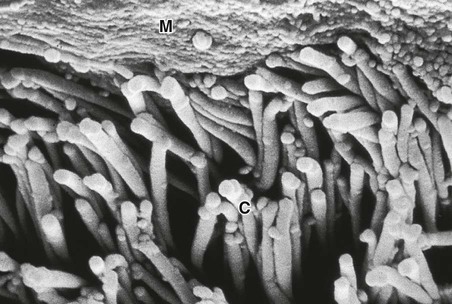
A The tapering, branching bronchus is armoured against compression by plates of cartilage. The more distal bronchioles are collapsible, but held patent by surrounding elastic tissue. B The unit of lung supplied by a terminal bronchiole is called an acinus. The bronchiolar wall contains smooth muscle and elastin fibres. The latter also run through the alveolar walls. Gas exchange occurs in the alveoli, which are connected to each other by the pores of Kohn. C Vascular anatomy of an acinus. Both the pulmonary artery (carrying desaturated blood) and the bronchial artery (systemic supply to airway tissue) run along the bronchus. The venous drainage to the left atrium follows the interlobular septa. From www.Netter.com – see p. 731.
Lung mechanics
Control of breathing
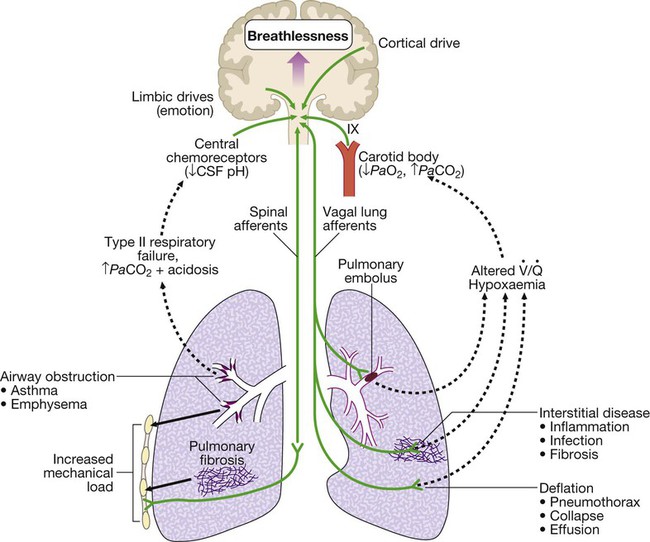
Mechanisms by which disease can stimulate the respiratory motor neurons in the medulla. Breathlessness is usually felt in proportion to the sum of these stimuli. Further explanation is given on page 543. (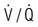 = ventilation/perfusion match)
= ventilation/perfusion match)
Ventilation/perfusion matching and the pulmonary circulation
Lung defences
Upper airway defences
Investigation of respiratory disease
Imaging
The ‘plain’ chest X-ray
The lung markings consist of branching and tapering lines radiating out from the hila. Where airways and vessels turn towards the film, they can appear as open or filled circles (see upper pole of right hilum). The scapulae may overlie the lung fields; trace the edge of bony structures to avoid mistaking them for pleural or pulmonary shadows. To check for hyperinflation, count the ribs; if more than 10 are visible posteriorly above the diaphragm, the lungs are hyperinflated. From Innes 2009 – see p. 731.
The dotted line in the drawings represents the normal position of the diaphragm. The dark pink area represents the extent of shadowing seen on the X-ray.
Computed tomography
Assessment of the pulmonary circulation
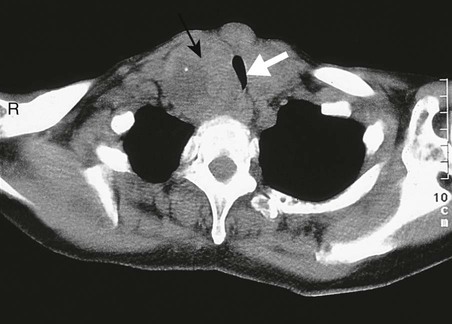
Positron emission tomography
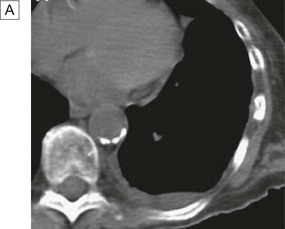
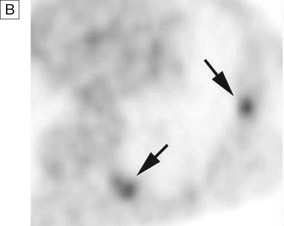
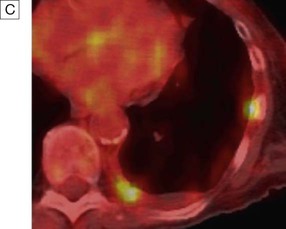
A In a patient with lung cancer, CT shows some posterior pleural thickening. B PET scanning reveals FDG uptake in two pleural lesions (arrows). C The lesions are highlighted in yellow in the combined PET/CT image. From http://radiology.rsnajnls.org – see p. 731.
Endoscopic examination
Laryngoscopy
Bronchoscopy
Immunological and serological tests
Microbiological investigations
Histopathology and cytology
Respiratory function testing
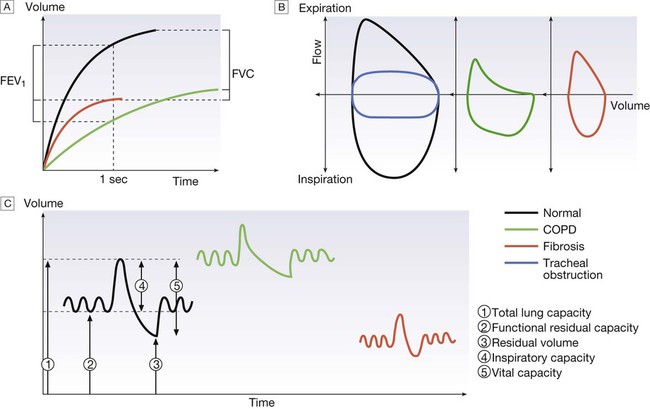
A Volume/time traces from forced expiration in a normal subject, in COPD and in fibrosis. COPD causes slow, prolonged and limited exhalation. In fibrosis, forced expiration results in rapid expulsion of a reduced forced vital capacity (FVC). Forced expiratory volume (FEV1) is reduced in both diseases but is disproportionately reduced, compared to FVC, in COPD. B The same data plotted as flow/volume loops. In COPD, collapse of intrathoracic airways limits flow, particularly during mid- and late expiration. The blue trace illustrates large airway obstruction, which particularly limits peak flow rates. C Lung volume measurement. Volume/time graphs during quiet breathing with a single maximal breath in and out. COPD causes hyperinflation with increased residual volume. Fibrosis causes a proportional reduction in all lung volumes.
Measurement of airway obstruction
Transfer factor
Arterial blood gases and oximetry
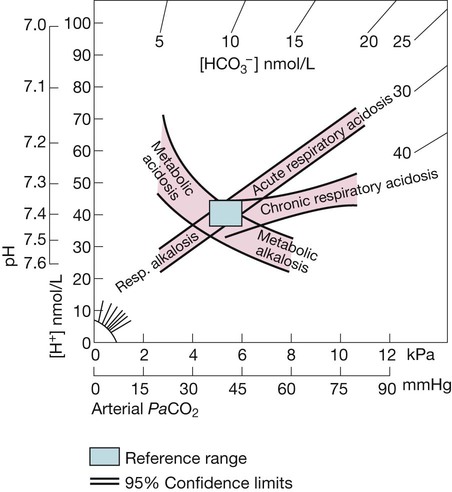
The rectangle indicates normal limits for [H+] and PaCO2. The bands represent 95% confidence limits of single disturbances in human blood. To determine the likely cause of an acid–base disorder, plot the values of [H+] and PaCO2 from an arterial blood gas measurement.. The diagram indicates whether any acidosis or alkalosis results primarily from a respiratory disorder of PaCO2 or from a metabolic derangement. Adapted from Flenley 1971 – see p. 732.
Presenting problems in respiratory disease
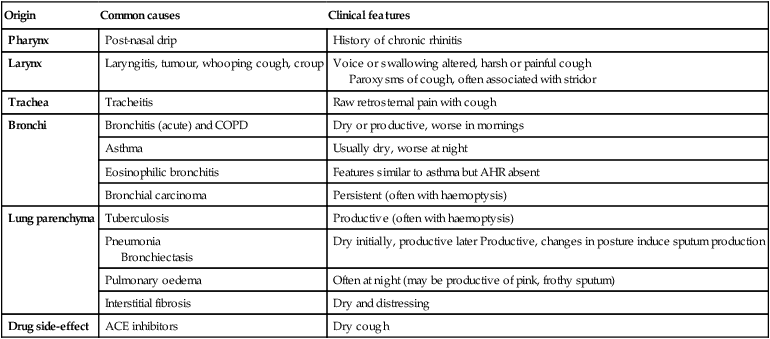
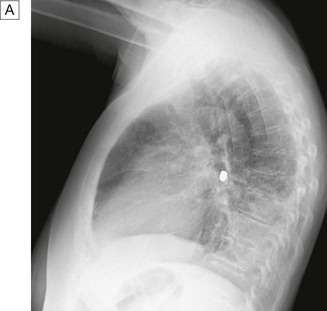
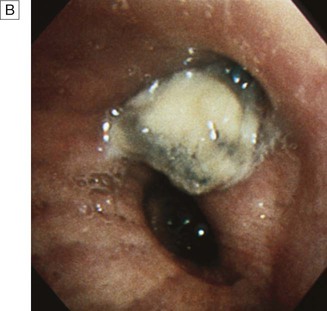
Cough
Causes of cough
Breathlessness
Pathophysiology
Chronic exertional breathlessness
Do you have other symptoms along with your breathlessness?
Acute severe breathlessness
History
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree