Fig. 11.1
Rectal dosage forms for systemic action: biopharmaceutical considerations
In contrast, very little is known about the biopharmaceutics of vaginal dosage forms. The vagina has good absorbing properties, but this is seldom used for a systemic effect. Vaginal dosage forms are administered for a local effect only.
Rectal and vaginal dosage forms aimed to obtain a local effect are, from a biopharmaceutical viewpoint, comparable with dermal preparations. However it should be known that after rectal and vaginal application a greater part of the active substance may reach the general circulation than after cutaneous application. This may result in significant blood levels and unwanted systemic effects.
Rectal administration of an active substance can be justified only if adequate data are available about the release from the dosage form and the absorption from the rectum. Without such a support rectal administration of an active substance should be discouraged. Tables with data from biopharmaceutical and pharmacokinetic research on rectally administered active substances are available from literature [5a].
11.3.1 Specific Problems of Rectal Administration
Compared to oral administration, rectal administration encounters some specific problems. Degree and rate of absorption of the active substance are more difficult to predict and depend largely on the never predictable residence time in the rectum. Both irritation of the rectal mucosa by the active substance or the excipients and a large (liquid) volume in the rectum may cause a defecation reflex terminating the absorption process of the active substance. Also the degree of filling of the colon and sometimes the rectum influences the release and the absorption of the active substance. In favour of rectal administration would be bypassing of the hepatic portal circulation and thereby the first-pass effect. However, clinical studies in men do not support this argument, see also Sect. 16.2.4.
Rectal absorption of an active substance proceeds, usually, more slowly and less completely than oral absorption. The active substance can only be absorbed after melting or dissolution of the base and dissolution of the active substance in the rectum fluid. These processes take time. Additionally, the surface for rectal absorption is much smaller than for oral absorption. Literature often advises higher standard dosages for rectal administration. The rectal dose of carbamazepine, for example, is for children 125 % of the oral dose [6]. No universal guidance could be found for the dose correction for rectal administration. When a rectal dosage cannot be found in literature the safest approach is to use the oral dosage.
11.3.2 Release and Absorption Rate
Contemporary research focuses on the development of dosage forms with a better and faster release of the active substance and on dosage forms with a delayed or controlled release. The addition of surfactants to the suppository often enhances the rate and extent of release and even the absorption of an active substance, but there are many exceptions. For a delayed or controlled release an increased viscosity of the suppository mass appears to be relevant. Most research has not yet yielded a licensed medicine.
Absorption Enhancers
Many studies have been published on the use of various enhancers for the rectal absorption. They increase the absorption of an active substance by enhancing the membrane permeation, rather than increasing the solubility. Published examples of absorption enhancers are capric acid and sodium caprate, lauric acid and sodium laurate, sodium salicylate and sodium cholate. These enhancers however give an unpredictable and strongly variable improvement of the biological availability [5b]. Nevertheless they sometimes lead to a licensed medicine: sodium caprate is already in use in a suppository product available in Japan [7].
Non-ionic surfactants can be added to a fatty suppository base to enhance the release of poor water-soluble active substances [5b]. The results of studies on this subject, however, vary considerably. Often the in vitro release is improved, whereas the in vivo results are disappointing [8a]. This is partly caused by the formation of micelles in the rectal fluid and partly by the influence of the surfactant on the rectum wall. In fat-based suspension-type suppositories the release rate is determined by the particle transport to the surface of the fatty phase and by the dissolution rate in the aqueous rectum fluid. For active substances with a moderate to poor solubility in water the rate-determining step is the dissolution of the active substance in the rectal fluid, see also Sect. 16.2.4. This dissolution may be accelerated by a better wetting of the active substance particles. This can be achieved by adding a surfactant [8b]. In general, the rate of release in vitro increases with an increasing amount of surfactant [8a]. However, in vivo there is a critical concentration. It appears that at higher concentrations of surfactant the absorption of active substance decreases again. The critical concentration of surfactant seems to be 1–3 % of the suppository weight. Probably the active substance gets enclosed in micelles in the rectal fluid. This may reduce or even impair the absorption [8a]. Surfactants may also change the membrane permeability. These changes depend on surfactant concentration and will alter the absorption. Because of such large and varying influences on the absorption of active substance, surfactants may only be added based on in vivo research, such as has been done with the influence of lecithin on absorption from indometacin suppositories. The influence of different concentrations of surfactant on release and absorption is illustrated with indometacin suppositories. Witepsol® H15 was used as suppository base and increasing amounts of lecithin were added. The addition of 1 % lecithin (about 25 mg per suppository) gave a distinctly higher blood concentration. The dissolution rate of indometacin was increased and, as a result of the decreased viscosity of the suppository mass by the surfactant, the suppository spread better in vivo, giving a larger area for indometacin release. In this way the surfactant increased dissolution over a larger area and hence increased the blood concentration [8a]. However, increasing the quantity of lecithin to over 300 mg per suppository produced slow release profiles and sustained plasma levels of indometacin when administered to rabbits. Consequently, a sustained release (controlled release) indometacin suppository was created [5c].
Controlled Release
Controlled release by increasing the viscosity of the suppository base has been studied with morphine.
A fatty base such as Witepsol® H15 is miscible with polyglycerol esters of fatty acids with a relatively high melting point, such as decaglycerol heptabehenate (HB750®) [9]. The increased viscosity of the melted mixture, results in a slower in vitro release of morphine sulphate compared to Witepsol H15 alone. In vivo the morphine level initially increases slowly and remains on a relatively high level for a longer time.
Morphine sulphate in a matrix of a fatty base (Witepsol® W25) with colloidal anhydrous silica (Aerosil® R 972 ) and hydroxypropylmethylcellulose (HPMC 4000) [10] showed an absorption rate and a biological availability equivalent to orally given morphine sulphate retard tablets (MS Contin®). Such suppositories can be made as a regular pharmacy preparation [11] but in our opinion this requires more convincing evidence that morphine release is sufficiently reproducible. MS-Contin® suppositories (not available in all countries) have a fatty base with sodium alginate and calcium phosphate [12].
Metoclopramide hydrochloride controlled release suppositories were prepared by mixing Witepsol® W35 with 30 % lecithin [13]. The metoclopramide is incorporated in this base in a (solid) reversed micellar solution. The diffusion rate of the active substance from the melted suppository in contact with the aqueous rectum fluid was very low. Compared to licensed normal metoclopramide suppositories a five times longer mean residence time was found in vivo for the lecithin suppositories.
A survey of research on special formulations of suppositories, such as hollow type suppositories, can be found in [5d].
Unconventional (Suppository) Bases and (Vaginal) Delivery Systems
Other rectal dosage forms may be used other than fat-based suppositories. One study describes a liquid ‘suppository’ that immediately after administration forms a gel with strong adhesion to the rectal mucosa [14]. The gelling at body temperature is caused by a poloxamer. Adhesion to the rectal mucosa is provided by carbomers and cellulose derivates. Compared to a fatty suppository, this delivery system is expected to spread less far into rectum and colon, thereby avoiding the hepatic portal system (see also Sect. 11.3).
The vaginal delivery system for dinoprostone (Propess® 10 mg) is even more different from the classical dosage forms for rectal or vaginal use. It is a thin, flat polymeric matrix of crosslinked macrogols forming a hydrogel in the vagina. Dinoprostone is released from the matrix in a controlled way: about 0.3 mg per hour during 24 h. A knitted polyester retrieval system envelopes the matrix and facilitates removal from the vagina at the time the cervical ripening has completed as decided by the gynaecologist [15].
Another intravaginal device is the vaginal ring see also Sect. 11.1. Already available is the NuvaRing®, used as contraceptive. It is a polymeric vaginal ring containing etonogestrel and ethinylestradiol. The ring has to be placed high in the vagina and should stay there for 3 weeks. After a 1-week break the next ring is inserted. Vaginal rings containing antiretroviral medicines for HIV prevention have been developed, but are not yet available for use. They show a sustained and controlled active substance release over 28 days, sometimes over 90 days. Polymers used for the rings are, for example, polysiloxanes and polyurethanes. For the NuvaRing® 2 different ethylene vinyl acetates are used.
11.4 Product Formulation, Suppositories
This section discusses the active substance (particle size and solubility), the bases and the excipients to obtain optimal release and absorption. Obviously these three components are closely interrelated. The active substance must be used in a chemical form, ionised or not, and with a particle size that are optimal for release and rectal absorption. In addition a base has to be chosen that optimises release, see also Fig. 11.1. Excipients can be added to improve dissolution and absorption or for technological reasons. However, excipients aimed at technological improvement may also influence the release of active substance. Whatever choice is made for active substance, base and excipients, their impact on the preparation process, the stability of the product and the shelf life must also be considered.
11.4.1 Particle Size of Active Substance
Usually the active substance does not dissolve in the base. Even lipophilic substances are often poorly soluble in fatty bases, so most suppositories are suspensions of the active substance in a (solid) vehicle, the suppository base. For these suspension suppositories the particle size of a dispersed active substance is important because it influences:
The pharmaceutical availability of the active substance
The physical stability of the suspension
The irritation of the rectal mucosa
The chemical stability of the active substance
The particle size is preferably chosen based on published data. If these are not available it has to be chosen in accordance with the physico-chemical properties of the active substance. Section 16.2.4 gives guidance for such a choice, summarised as follows:
For a fatty base with a good water soluble active substance, a particle size as large as possible should be taken, but not above 180 μm.
For a fatty base with a slightly water soluble active substance that is very slightly or practically insoluble in the fatty phase as well, a particle size as small as possible should be taken, for instance 45 μm for paracetamol.
For a fatty base with a slightly water soluble active substance that is sparingly to freely soluble in the fatty phase, the particle size of the active substance has hardly any influence.
For a water soluble base with a slightly water soluble active substance, small particles are chosen because they dissolve more readily; usually a particle size not exceeding 180 μm satisfies.
A stable suspension of active substance in the base is important during the preparation process, especially when the melted mass is poured into the molds and during subsequent cooling and solidification. The smaller the particles, the more stable the suspension (see also Sect. 18.4.2). In practice, a particle size not exceeding 180 μm meets all demands. Any agglomerates should be carefully fragmented.
Irritation of the rectal mucosa occurs when large particles are used. A particle size of 180 μm should therefore be the maximum. Irritation of the mucosa may also occur when small particles of an irritating active substance dissolve (too) quickly. This happens for instance when acetylsalicylic acid with a particle size of 45 μm is used in suppositories. It has a fast release of the active substance from the suppository and it dissolves rapidly, but as a result it irritates the mucosa. Therefore acetylsalicylic acid should be used with a particle size of 180 μm.
Chemical degradation occurs faster when the total surface of the particles is larger. Therefore a larger particle size enhances chemical stability, for instance particles of 90–180 μm.
11.4.2 Solubility of Active Substance
If an active substance is practically insoluble in water (and therefore in the rectal fluid), the suppository will be ineffective. Certain excipients may increase the water solubility and, as a result, the absorption. A surfactant can be added for a better wetting of the particles and thereby increasing dissolution rate. A macrogol (polyethylene glycol) suppository base may increase the solubility of the active substance by acting as a co-solvent. As the suppository dissolves in the rectal fluid, a mixture of macrogol and rectal fluid develops, in which the active substance may dissolve better.
The active substance should have certain lipophilicity as well. If not, it will not pass the rectal lipoid membranes. On the other hand, a too high lipophilicity causes the active substance to be hardly released from a fatty base. For a systemic effect a good water-soluble active substance is preferred with sufficient lipophilicity at the pH of the rectal fluid to allow passage of the rectal membranes for absorption [8c].
Which form of the active substance should be chosen, more hydrophilic or more lipophilic, is related to the choice of the suppository base, fatty or water soluble. These choices can only be based on published data about release and absorption.
Form of Active Substance and Type of Base in Relation to Efficacy
Glafenine has a solubility in water of 1 in 60,000 at pH 7. The rectal absorption of glafenine from an aqueous micro-enema, and of glafenine hydrochloride from a fatty suppository is extremely slow and incomplete, due to the low solubility of glafenine at the pH in the rectum [16]. Therefore this active substance is not effective after rectal administration, although glafenine suppositories were commercially available in the past century. On the contrary, for example, the solubility of phenobarbital in water of 1 in 1,000 is sufficient for rectal administration.
Hydrocortisone (soluble in water 1 in 3,500) is better soluble than hydrocortisone acetate (practically insoluble in water). Therefore, in a suppository given for a systemic effect, hydrocortisone is used, but in a suppository given for local problems such as haemorrhoids, hydrocortisone acetate is preferred, in order to avoid unwanted systemic effects.
Phenobarbital sodium can be processed in a fatty base and the release of this salt is faster than that of the corresponding acid, phenobarbital. The bioavailability of both substances is almost equal, but for the rapid increase of plasma concentrations the salt is the better choice [17, 18].
Sodium valproate is released well from a fatty base, but absorption is better with the free acid in a fatty base [17]. Moreover, the processing of valproic acid is much easier than that of the strongly hygroscopic sodium salt. For both reasons the acid is preferred.
Methadone is best incorporated in a macrogol base in the form of its hydrochloride salt, to obtain a good efficacy. Methadone hydrochloride (pKa 8.25) will be released from a fatty base, but at the rectal membrane methadone is converted to its unionised form. This lipophilic substance is so poorly soluble in the aqueous rectal fluid that it is not absorbed. By using a macrogol base (macrogol 1500 or PEG 1500), the solubility of the unionised methadone in the rectal fluid (now containing the macrogol as co-solvent) is improved [19, 20].
11.4.3 Types of Suppository Base
The common suppository bases can be classified into two main categories: the lipophilic and the hydrophilic bases. See Sect. 11.3 and Fig. 11.1 for the choice between these types from a bioavailability viewpoint. Usually a fatty base will comply but a lipophilic active substance may not be released well enough, because the partition coefficient lipid/water is unfavourably high. The consultation of published data about active substance release and absorption is recommended. When published data are not available, the solubility of the active substance (Sect. 11.4.2) should be considered, but without in vivo test the therapeutic action is not assured. This section considers some general characteristic points of the bases and gives some information on the less usual bases cocoa butter and glycerinated gelatin. The most used bases, hard fat and macrogol, are dealt with in Sects. 11.4.4 and 11.4.5.
A suppository base should not irritate the rectum and be harmless in case of absorption. Chemically and physically, the base should have a good shelf life and not decrease the stability of the active substance.
With regard to the preparation process the following requirements are set:
Upon solidification, the base must not form unstable modifications with a solidification point below room temperature, as is seen for cocoa butter.
Upon solidification, the suppository mass (base + active substance(s) + excipient(s)) preferably contracts slightly, facilitating removal from the mold.
At the pouring temperature, the suppository mass should be sufficiently viscous to prevent the settling of dispersed particles during the filling process.
At room temperature, the suppository mass must be solid and must keep its shape.
Seldom will a suppository base meet all these qualities. The properties of a base may be improved by the addition of a substance that influences the melting point. For instance, the use of a macrogol (polyethylene glycol or PEG) combination with a higher melting point than the usual macrogol base gives chloral hydrate suppositories a sufficient consistency (Table 11.1).
For a suppository mold 2.3 mLa: | |
Chloral hydrate | 0.5 g |
Macrogol 1500 | 2 g |
Macrogol 6000 | 0.17 g |
A low melting point may be necessary when the body temperature of the patient is below normal. A low body temperature may occur at terminal illness. From a standard suppository base, the release of the active substance will be insufficient. For a low melting point, 20 % of the fatty base is replaced by a liquid triglyceride such as Miglyol®, such as is done for morphine suppositories in clinical practice [22]. A low melting point may also be needed to obtain a better local effect, for example in 10 % zinc oxide suppositories (Table 11.2) that are used to soothe haemorrhoidal pain. In this formulation 20 % of the total weight consists of Miglyol® 812. As a result of the low melting point, the suppository melts superficially at insertion, creating a protective layer in the anal canal.
For a suppository mold of any volume: | |
Zinc oxide (90) | 10 g |
Triglycerides, medium-chain | 20 g |
Hard fat | 70 g |
Total | 100 g |
Increasing the melting point of a fatty suppository above a normal body temperature is not an option. Not even in suppositories for tropical use if melting during storage and transport has to be prevented. With a higher melting point the in-patient melting time and therefore the time to the release of the active substance will be unacceptably long.
11.4.3.1 Cocoa Butter
Cocoa butter (Cacao oleum) is a solid fat, pressed from the roasted seeds of Theobroma cacao. Since the middle of the eighteenth century it is used as a suppository base. Cocoa butter melts at body temperature. At room temperature it is a solid mass. But cocoa butter has a number of undesirable properties: it soon becomes rancid on storage and it exhibits marked polymorphism. It has four polymorphic forms: alfa, beta, beta-accent and gamma. The melting points are 22 °C, 34–35 °C, 28 °C, and 18 °C respectively. The beta-form is the most stable one.
Cocoa butter suppositories can be extemporaneously prepared by hand rolling (the oldest, historical method), compression molding (compressing cold mass into the molds) and fusion molding (filling the molds from a melt). The first and the second method have the advantage of avoiding heating and melting of the cocoa butter. Problems on solidification of the mass, caused by the polymorphism, are bypassed in this way. In both methods grated cocoa butter is mixed with the active substance in a mortar, using a plastic card. In hand rolling the mass from the mortar is pressed in the palm of a hand into an elastic mass that is cut into an appropriate number of portions and subsequently rolled by hand on a flat surface to obtain a cylindrical shape, conical at one end. Plastic gloves should be worn when forming the suppositories. Hand-operated compression molds facilitate different shapes and sizes of the suppositories, so there is little need for shaping by hand anymore [5e].
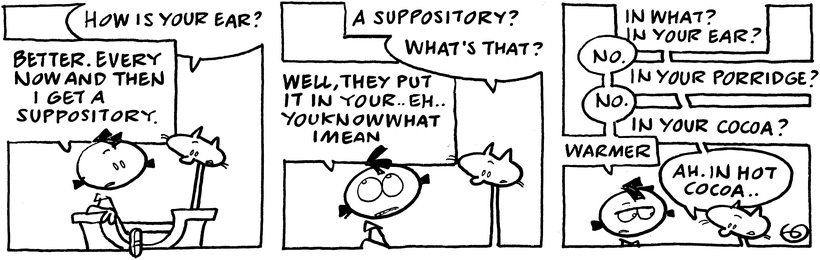
In compression molding, the mixture in the mortar is transferred to special pressing equipment, holding the proper compression mold (see Fig. 11.2). The method requires a replacement factor (see Sect. 11.5.1) specifically valid for this compression method. In both ‘cold’ methods the quality of mixing active substance and grated base is very critical for sufficient content uniformity of the suppositories. Although no investigations are available, it is obvious that this process is most critical with low-dosed active substances and definitely has to be validated.
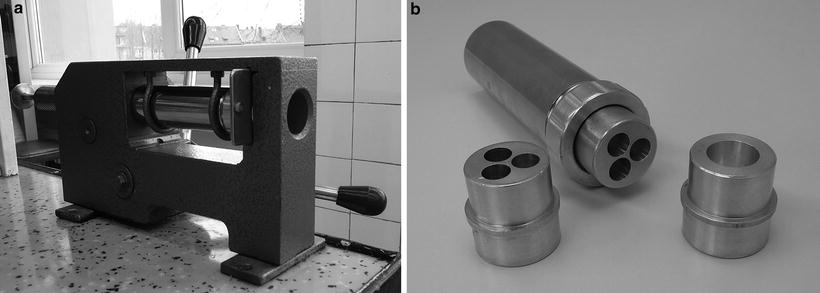

Fig. 11.2
Hand press (a) and molds (b), used in compression molding. Source: Department of Pharmaceutical Technology GUMed Gdansk, with permission
Fusion molding involves mixing the active substance with molten cocoa butter and pouring the mixture into the mold. Because of the polymorphism, great care is needed to melt the base properly. When cocoa butter is melted too fast and too far above 36 °C is kept too long too warm or is cooled down too quickly, the result is the metastable alfa-modification. This modification has a substantially lower melting point giving rise to difficulties in solidification. In fact, the melting point may become so low that the cocoa butter will not solidify at room temperature any more. However there is a slow transition from the alfa-form to the more stable beta-form (with higher melting point), which may take several days [5f]. An ongoing transition of unstable to stable modifications during storage may affect the melting properties of the suppository and therefore the release of the active substance.
As opposed to these disadvantages of cocoa butter in fusion molding, there is the advantage that in compression molding (cold preparation) no settling of particles will occur, thus eliminating a main cause for content uniformity problems.
Hard fat, the alternative for cocoa butter, is less sensitive to rancidity and shows substantially less modifications. Therefore it has replaced the cocoa butter as a suppository base in Dutch pharmacies, although the disadvantage of particle settling during pouring has to be overcome instead. Hard fat is the main suppository base in many countries. Cocoa butter has until now a place in suppository preparation in some other countries.
11.4.3.2 Glycerinated Gelatin
The oldest water-soluble suppository base is a mixture of gelatin, glycerol and water. The ratio differs from country to country. The Dutch pharmacopoeia (Ph. Ned. VI) specified Suppositoria Gelatinosa as containing 2 parts of gelatin, 5 parts of glycerol 85 % and 4 parts of water. BP and USP describe this type of base as well. The glycerinated gelatin base has long been used in pessaries (vaginal suppositories) for its good adhesion properties to the vaginal mucosa and its patient friendly softness. However, a glycerinated gelatin base may give microbiological problems on storage. Using a natural starting material (gelatin) enhances the chance of microbiological contamination. Water can condense at the package and micro-organisms may then grow. Physical problems related to storage are loss of water in dry circumstances and a tendency to absorb moisture under damp conditions. For these reasons and from the authors’ viewpoint, the use of glycerinated gelatin base for rectal and vaginal dosage forms is obsolete. Nevertheless, in many countries this base is used for pessaries. Such pessaries must be kept in well-closed containers and in a cool place. For an extended shelf life a preservative should be added, such as methyl parahydroxybenzoate or propyl parahydroxybenzoate or both (see Sect. 23.8.5). Otherwise the shelf life should be limited to 1 or 2 weeks.
11.4.4 Hard Fat (Adeps Solidus)
Hard fat is a collective name for (semi-)synthetic mixtures of mono-, di- and triglycerides of the fatty acids C10 – C18 with a melting range between 33 and 36 °C. It is marketed in many varieties and under various brand names. An elaborate survey with the composition, melting and solidification range, hydroxyl value, acid value, saponification value, peroxide value and iodine value can be found in [5g]. Table 11.3 shows the characterisation parameters of common fats used as suppository bases. Some of these properties will be described.
Table 11.3
Characterisation parameters of commonly used lipophilic suppository bases
Suppository base | Melting range (°C) | Solidification range (°C) | Hydroxyl valuea | Iodine valueb | Acid valuec |
|---|---|---|---|---|---|
Adeps solidus (hard fat)d | 30-45 | – | ≤50 | ≤3 | ≤0,5 |
Novata B | 33.5–35.5 | 31–33 | 20–30 | ≤3 | ≤0.3 |
Suppocire AM | 35.0–36.5 | – | 5 | – | – |
Witepsol H15 | 33.5–35.5 | 32.5–34.5 | 5–15 | ≤3 | ≤0.2 |
Witepsol W25 | 33.5–35.5 | 29–33 | 20–30 | ≤3 | ≤0.3 |
Witepsol W35 | 33.5–35.5 | – | 40–50 | ≤3 | ≤0.3 |
Witepsol E75 | approx. 38 | – | ≤15 | ≤3 | ≤0.3 |
Cocoa butter | 31–34 | – | approx. 0 | 31–38 | <5 |
11.4.4.1 Hydroxyl Value
By varying the ratio of mono-, di- and triglycerides, different hydroxyl values are obtained. A higher hydroxyl value is, among others, associated with more tendency to further hardening after preparation and a higher elasticity and viscosity of the (molten) base [8d]. Higher elasticity diminishes the risk of fracturing at rapid cooling. A higher viscosity is advantageous for the suspension stability of the active substance in the molten base, but may also reduce the availability of the active substance [24]. Otherwise, a very low hydroxyl value may have an unexpected influence on the active substance release. Therefore bases with very different hydroxyl values should not be exchanged without further investigation.
11.4.4.2 Acid Value
A low acid value is desirable because irritation of the mucous membrane and chemical reactivity increase with a higher content of free fatty acids. According to the Ph. Eur., the maximum acid value is 0.5.
11.4.4.3 Iodine Value
The iodine value is a measure of the number of double bonds in an oil or a fat. A low iodine value in the base is achieved by using esters from saturated fatty acids. A large number of double bonds increases the tendency to oxidation and rancidity.
11.4.4.4 Peroxide Value
The peroxide value is a measure of the amount of reactive oxygen in the fat. A low peroxide value decreases oxidation of the active substance by the base which enables processing of easily oxidisable active substances, such as ergotamine and chlorpromazine.
11.4.4.5 Saponification Value
The saponification value is directly related to the molecular weight of the fats, and thus to the chain length of their fatty acids and to the melting point. Hard fat with a melting point (actually a melting range) between 33 and 36 °C meets the requirements of the Ph. Eur. regarding the saponification value of 210–260.
The requirements set by the Ph. Eur. to hard fat are relatively broad; all mentioned brands meet them. To limit formulation variation, it is recommended to use only one or two types of fatty suppository bases. Commonly used are Witepsol ® H15, Suppocire ® AM, Novata ® B and Witepsol W25. Witepsol H15 is the most commonly used suppository base in the Netherlands and many other countries. Suppocire AM is useful for acidic ingredients. Its low hydroxyl value may diminish interaction between free hydroxyl groups and acidic active substances. The last two bases may withstand forced cooling, as they have a higher hydroxyl value and consequently a larger elasticity [25].
11.4.4.6 Solidification Point or Solidification Range
The Ph. Eur. sets no requirements for the solidification point or range of hard fat. Hard fat types which meet the requirements of Ph. Eur. and have the same melting range, may still have different solidification ranges due to a different composition.
A short interval between melting point and solidification point seems to be advantageous as it shortens the time that particles can settle after the suppository mass has been poured into the molds. This settling may lead to a brittle tip. This is however not necessarily true, because often supercooling occurs before the mass starts to solidify [8e]. Forced cooling will shorten the solidification time but it cannot be applied to each type of hard fat; fractures easily occur in the suppository.
A different composition of hard fat also influences the solidification behaviour and may cause a (welcome) increase of viscosity before the mass solidifies completely. The viscosity increase may decrease settling during the filling process and thereby improve content uniformity. Often however, during cooling, no increase of viscosity is seen but suddenly a fast solidification occurs. This solidification behaviour complicates manual preparation of suppositories. The lack of gradually increasing viscosity is a quality aspect of hard fat that usually gets too little attention.
As for cocoa butter various modifications exist for hard fat, but their consequences are much less pronounced [8f]. Therefore, control of temperature during melting and cooling comes far less precise than in cocoa butter. Nevertheless, the polymorphism of hard fat is responsible for some physical processes including the process of secondary hardening (ageing) as described in Sect. 11.4.8. It is hardly possible yet to trace back this process to certain phase transitions.
11.4.4.7 Compatibility
Hard fat is compatible with the majority of active substances. However, active substances with free acid groups can react with the free hydroxyl groups of the mono- and diglycerides. For such active substances a base with a low hydroxyl value should be used.
Active substances can also react with the free fatty acids in the base. To avoid this, a base with a low acid value can be chosen, so with few free fatty acids.
Reactions of an active substance with free hydroxyl groups or free fatty acids of the base changes the melting behaviour and thereby may affect the release of the active substance.
Reactions of an active substance with peroxides in the base can be prevented by choosing a base with a low peroxide value. When formulating ergotamine or chlorpromazine, the peroxide value of the base must not exceed 0.5.
11.4.5 Macrogol
Macrogols are polymers of ethylene oxide, see Sect. 23.3.4. Usually mixtures of macrogols are used as suppository base. The desired hardness can be adjusted by choosing the molecular weight and suitable ratios. Even macrogol 1500 is too soft if used as such [8g]. The most commonly used base in Dutch pharmacies is 1 part of macrogol 1500 plus 2 parts of macrogol 4000. If suppositories with this base become too soft, a macrogol with a higher molecular weight should be used, see the example in Table 11.1.
11.4.5.1 Advantages and Disadvantages
A macrogol base will mostly be chosen based on published data showing better release or absorption, or both. The advantage of water solubility and thus avoidance of the melting time is somewhat overestimated because the dissolution of macrogol in the rectal fluid takes time as well. Once the macrogol has been dissolved the active substance release is faster than from a fatty base because in the latter transport of the active substance from the fatty to the aqueous phase still has to take place. Moreover the active substance can dissolve in the rectal fluid simultaneously with the base. The base has considerable disadvantages however: chemical incompatibilities and irritation.
11.4.5.2 Incompatibilities
Macrogols are incompatible with many more active substances, compared to hard fat. Upon storage, macrogols can form peroxides and therefore they are not suitable for the processing of easily oxidisable substances such as ergotamine and chlorpromazine (Sect. 22.2.2). Incompatibilities may also be caused by complex formation and trans-esterification. Acetylsalicylic acid decomposes rapidly in a macrogol base, yielding salicylic acid while macrogol is acetylated. A list of incompatibilities with macrogols can be found in [26]. Because of its high melting point, a macrogol base is often recommended for use in tropical circumstances. Its usability is however restricted by the chemical incompatibilities and the hygroscopic character.
11.4.5.3 Irritation
From a macrogol base the active substance is released not only by melting of the base, but primarily by dissolving of the base in the rectal fluid. Due to the high osmotic value of the dissolved macrogol, water is withdrawn from the surrounding tissue. This may cause irritation [8g]. Moistening the suppository with water before insertion is suggested to avoid irritation [5h]. It will help insertion but the effect on irritation is questionable because much more water will be attracted when the suppository dissolves.
11.4.6 Excipients
In suppositories all excipients should be used with care. The addition of excipients often negatively influences active substance release. This applies for instance when tablets are used as starting material because of costliness or unavailability of the active substance as such or when the quantity needed is too small to be weighed with sufficient accuracy (see Sect. 29.1.8). The very nature and the amount of excipients in tablets is usually unknown and may have unexpected effects on active substance release. Especially talc and magnesium stearate, common excipients in tablets and insoluble both in fat and in water, may strongly impair the release by accumulating in the interface between fat and water [24].
11.4.6.1 Lactose
In suspension suppositories, the active substance is processed as small particles that are prone to form agglomerates. The effective dispersion of the agglomerates (see Sect. 29.3) is a prerequisite for a sufficient content uniformity. Lactose may be used in pharmacy preparations to disperse the agglomerates and to maintain separation of the primary particles. This is most important with low dosed active substances, which do not easily lead to a good content uniformity. If 50 mg or less of active substance(s) is used per suppository, as a standard 100 mg lactose may be added to each suppository, as illustrated by chlorpromazine suppositories in Table 11.4. The added lactose should not be considered as a filler only as it is in tablets or capsules, but its main function in suppositories is the dispersing of the active substance.
Chlorpromazine hydrochloride | 0.025 g |
Lactose monohydrate (180) | 0.1 g |
Hard fat with peroxide value ≤ 0.5 | q.s. |
In large-scale preparations of suppositories lactose may be added for another purpose. Sometimes a high dose analgesic suppository has a good structure, while a low dose suppository is brittle. In such case another suppository base may be used. More easily, the volume of the suspended active substance may be increased by adding lactose. This provides a good suppository too. In this case the lactose only acts as filler. Other fillers used in suppositories are sucrose and microcrystalline cellulose [8h].
11.4.6.2 Colloidal Anhydrous Silica
Dispersing of agglomerates may also be achieved by using colloidal anhydrous silica (colloidal silicon dioxide use Aerosil® 200V, the compressed quality). For example, colloidal anhydrous silica facilitates the dispersing of agglomerates in a mixture of paracetamol and codeine phosphate. It should be added in an amount of 0.5 % of the amount of paracetamol, as in suppositories with high doses of paracetamol and codeine (Table 11.5). In general, colloidal anhydrous silica can be added up to a maximum of 1 % of the weight of the active substance.
For a suppository mold of at least 2.8 mL: | |
Paracetamol (45) | 1 g |
Codeine phosphate hemihydrate (90) | 0.06 g |
Silica, colloidal anhydrous (compressed) | 0.005 g |
Lecithin (NF) | 0.06 g |
Hard fat | At least 1.8 g |
When silica is used, it should be considered that the release of aqueous soluble to very soluble active substances may be reduced. Silica increases the viscosity of the base which may impair the transport of the active substance to the interface between water and fat, thereby impairing active substance release. For water-soluble active substances, crossing the interface is rate determining (Sect. 16.2.4). For this reason the amount of silica has to be limited to 1 % of the weight of the active substance. A negative influence of anhydrous colloidal silica on active substance release is evident for amounts above 1.5–2 % of the suppository base [8i]. Concentrations above 1.5–2 % are used only for suppositories with an intentionally delayed release profile.
11.4.6.3 Lecithin
When large amounts of active substance are incorporated in a suppository, especially when the active substance particles are very small, the viscosity of the melted mass may become so high that it cannot be poured out into the mold. This happens in the preparation of suppositories containing 1,000 mg paracetamol with a particle size of 45 μm and Witepsol H15 as the base (Table 11.5). Adding a small quantity of soya lecithin renders the mass more fluid. In general, 1–2 % of soya lecithin, calculated on the suppository weight, is sufficient [8h], see Table 11.5. A disadvantage of adding soya lecithin is that air gets beaten into the suppository mass more easily [28]. Larger amounts of lecithin may delay the absorption of the active substance (see Sect. 11.3.2).
11.4.6.4 Antioxidants
In pharmacy preparations of suppositories antioxidants are seldom used. If needed, butylhydroxytoluene (BHT) and butylhydroxyanisole (BHA) may be the right choice in fatty suppositories, but see Sect. 22.2.2. These antioxidants are lipophilic and dissolve easily in a fatty base. The usual concentration in fats is 0.02 % [30], see also Sect. 23.9.
11.4.7 Shape and Size of Suppository Molds
Suppositories may appear in various shapes, including a cylindrical, a conical and a torpedo form. The torpedo shape is generally used in pharmacy preparations, see Fig. 11.6. Suppository size varies from 1 to 4 mL. Commonly a size of 2–3 mL is used, for young children usually a size of 1 mL is taken, see Table 11.6.
Table 11.6
Suppository Molds
Volume | Application |
|---|---|
1.15 mL | Children up to about 4 years |
2.3 mL | Standard |
2.8 mL | Larger quantities of active substance |
From a biopharmaceutical viewpoint, a large sized suppository may be advantageous. In principal, a larger volume spreads over a larger intestinal surface. As a result more active substance is released from the base, more active substance dissolves in the rectal fluid and the absorption is faster. This is especially important for substances that are poorly soluble in both fat and water, such as paracetamol. The release of active substance from the base in this case strongly depends on the extent of the interface between fat and rectal fluid available for active substance release. From a technological point of view a large size suppository must be chosen if a large amount of active substance, such as 1,000 mg paracetamol, has to be incorporated. Even using a 2.8 mL mold, the viscosity of the suppository melt is so high that the addition of lecithin is necessary to allow pouring of the melt into the molds (see Table 11.5).
11.4.8 Stability
Chemical and physical processes may limit the stability of active substance, base and excipients in suppositories. Also the packaging and the conditions and times of storage need attention. Suppositories with a fatty or a macrogol base do not contain water. Therefore bacterial growth is unlikely and preservation is not needed.
11.4.8.1 Chemical Stability
Modern suppository bases usually do not contain water. Hydrolysis of an active substance will therefore seldom be an issue. Oxidation, in contrast, occurs. Oxidative reactions occur in the presence of oxygen and peroxides, under the influence of light and heat and are catalysed by traces of heavy metals, see Sect. 22.2.2. Macrogols (see Sect. 11.4.4) and some types of hard fat may form peroxides under influence of light, air and heat. These peroxides may readily oxidise oxidisable substances such as ergotamine and chlorpromazine which should, therefore, be incorporated in a hard fat (with a low peroxide value). Storage in the refrigerator (2–8 °C) will increase storage times, for example of suppositories with ergotamine and chlorpromazine hydrochloride (see Tables 11.7 and 11.4). Both active substances have to be protected from light as well by choosing an appropriate packaging. Chemical degradation by oxidation increases proportionally with the particle surface area in contact with the base. Smaller particles of an active substance will therefore oxidise more rapidly.
Caffeine | 0.1 g |
Ergotamine tartrate | 0.00105 g |
Tartaric acid | 0.002 g |
Hard fat with peroxide value ≤0.5 | q.s. |
11.4.8.2 Physical Stability: Crystallisation of the Active substance
Active substances, and especially those that are partially soluble in the fatty suppository base, may crystallise during storage. Heating the suppository mass before or in the filling process may lead to a partial dissolution of the active substance in the base. A supersaturated solution will be formed on cooling down, with, as a consequence, re-crystallisation and crystal growth during storage (see Sect. 18.4.2.3). This makes particle size uncontrollable. In a macrogol suppository base partially dissolved indometacin may recrystallise at storage, which becomes visible as a spotted appearance. In a fatty base an active substance such as valproic acid dissolves into a higher amount at the preparation process than at storage at room temperature. It is recommended to add this kind of substances to the molten base immediately before the filling process, meaning at the lowest temperature of the melt. The rate of solidification also may influence the physical stability. The longer the solidification process of fatty valproic acid suppositories, the more the valproic acid separates from the solution in visible structures. This may even lead to a ‘wet’ and grainy suppository tip. Therefore the valproic acid suppositories (Table 11.8) are placed in the refrigerator immediately after the filling process.
For a suppository mold of at least 2.8 mL: | |
Valproic acid | 0.5 g |
Hard fat | q.s. |
In most other suppositories rapid solidification, due to placement of the suppositories into the refrigerator, leads to faults and fractures in the suppositories and should therefore be avoided.
Control of Chemical and Physical Stability, an Example
Indometacin is often formulated in a macrogol base to get a good biological availability [33]. In that base discoloration and esterification may easily occur. Furthermore, the indometacin slowly crystallises giving the suppository a spotted appearance. The antioxidants butyl hydroxytoluene (BHT), butyl hydroxyanisole (BHA) and disodium edetate are added to improve the stability as well as glycerol to limit crystallisation, for instance in Indocid® suppositories (Aspen, UK) [34]. Nevertheless, a fatty base must be preferred. Indometacin is released almost equally from a fatty base as from a macrogol base [8a, 35, 36] and the chemical and physical stability is better in fat. For instance indometacin suppositories (Centrafarm, Netherlands) [37] and indometacin suppositories BP (Actavis, UK) are manufactured with hard fat without additives [34].
11.4.8.3 Physical Stability: Hardening
Further hardening of suppositories on storage (secondary hardening or ageing) may change the crush strength (resistance to crushing) and the melting behaviour. This may be relevant for the therapeutic effect [38, 39]. The secondary hardening process of the suppository base is caused by a further conversion from the liquid phase to the solid phase and by a slow transition into modifications with a higher melting point. These changes usually cause crush strength to increase. They also lengthen the melting time, resulting in a slower release of the active substance. Secondary hardening of suppositories may be limited by cool storage, after sufficient initial hardening. Therefore, suppositories should generally be stored in a cool place.
11.4.9 Packaging
For the packaging of suppositories the chemical and physical properties of the active substance and the base have to be considered. This should be done with respect to sensitivity to oxygen and light, compatibility with the (plastic) package, material evaporation (chloral hydrate) and hygroscopicity (macrogol and different active substances).
Suppositories are preferably dispensed in the plastic disposable molds in which they have been filled (see Sect. 24.4.10). The molds are placed in a carton. An alternative is a well-closed container, a glass or plastic jar, protecting the content against the influence of light and air (see Sect. 24.4.7). Such a jar is needed for instance for suppositories with a macrogol base, such as those with chloral hydrate (Table 11.1). These hygroscopic suppositories remain dry in the plastic strip (mold) placed in a glass jar, while in the plastic strip placed in a carton they become wet. Even with no plastic strip and placed in a glass jar these suppositories become wet and stick together. The upper surface of the plastic strip with macrogol suppositories may be taped before they are placed in a carton, but this is not always sufficient to prevent the suppositories getting wet. Suppositories that are not molded in a plastic strip should always be packed in a light and air resistant jar. For arthritic patients it is impossible to open plastic strips, which makes dispensing the suppositories out of the strip in a glass or plastic jar necessary.
11.4.10 Storage
Shelf life of pharmacy preparations depends on the character and stability of the preparation and has a maximum of 3 years as is explained in Sect. 22.7.1. Generally, physically and chemically stable suppositories have an expiry date of 3 years after preparation, both in strips in a carton or without strip in a jar. It may be convenient to split up this period in 24 months in the pharmacy and 12 months for use by the patient.
Suppositories prepared as extemporaneous preparations following a non-standardised formula, having an unknown chemical or physical stability, should immediately be dispensed to the patient who gets a maximal usage period of 1 month only (see Sect. 22.7.2) if classified as semisolid preparation. If the patient only takes a suppository ‘on demand’ a usage period of 1 month is very short and it may be justified to consider them to be comparable to a dry dosage form with an arbitrarily chosen maximum usage period of 6 months.
For standard preparations the storage conditions and the maximum shelf life are determined in the design phase and stated in the formulary.
11.4.11 Labelling
Section 37.3 gives the general requirements for labelling. There should be a clear indication how to use the dosage form, for instance ‘for rectal use’ or ‘(rectal) suppositories’ as well as how to take a suppository out of the plastic strip. This requires some expertise and strong hands as well.
The correct way to use and insert the suppository should be explained in a patient information leaflet. A Dutch leaflet advises the patient to insert the suppository while lying in a lateral position with the upper knee flexed. After the suppository has been inserted the patient should stay in the horizontal position for 5–10 min, if possible. Another position is bending forward or squatting (sitting on the heels) while inserting the suppository. The patient should insert the suppository (torpedo) tip into the anus and then push the suppository with his finger until that finger is about 2 cm in the anus (for children 1 cm). However, insertion of the suppository with the blunt side forward is reported to have real advantages [40]. There is no need to introduce a finger into the anal canal. The external anal sphincter constricts physiologically better around the tip of the suppository and following this method the patient will better retain the suppository. It is also not necessary to stay horizontal after insertion.
Whatever the method of insertion, the instruction should mention that the rectum is better empty before insertion and thus free from stool. The patient should go to the toilet first if necessary.
To minimise the burning sensation of macrogol suppositories it is often suggested to moisten the suppository prior to insertion. The authors do not expect this will prevent irritation of the mucosa, because the macrogol attracts much more water after being inserted than supplied by moistening. But it might facilitate insertion of the suppository (Sect. 11.4.5).
11.5 Method of Preparation, Fat-Based Suspension Suppositories
The method of preparation particularly depends on the physico-chemical form of the suppository: a solution or a suspension of the active substance in the base. The nature of the base is of less importance for the preparation method. The most frequently occurring suppository is the fat-based suspension-suppository. The preparation of a suspension suppository is more critical than a solution suppository and therefore requires special attention.
First the calculation of the required quantity of base and the necessary excess is dealt with. Next the preparation steps, dispersing, mixing and pouring, are discussed. These steps are important for a homogeneous distribution of the active substance over the suppositories, its content uniformity (see Sect. 32.7.2). For a homogeneous distribution the so-called squeeze bottle method, has proven to be more effective than more well-known methods. In this method a plastic bottle with a straight nozzle is used, called squeeze bottle. Figure 11.4 surveys suitable combinations of dispersing and pouring methods for small batches of suppositories [42]. The last processing step includes cooling and finishing the suppositories.
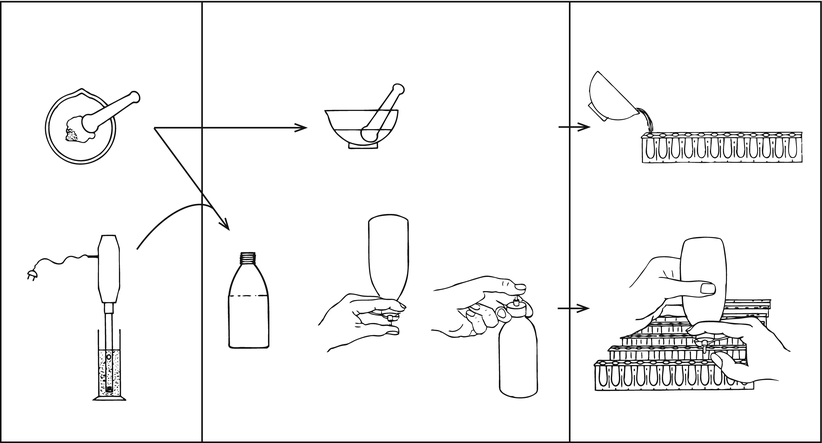

Fig. 11.4




Combinations of dispersing, mixing and pouring methods in the preparation of small batches of suppositories. The bottle used for pouring is a plastic bottle with a straight nozzle (Sect. 11.5.5), called squeeze bottle. Source: Recepteerkunde 2009, ©KNMP
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree