Minimal temperature °C
Maximal temperature °C
Long time
Short time
Long time
HDPE
−50
90/120
70/80
LDPE
−50
80/90
60/75
PET
−20
80
65
PP
0/−30
140
100
COC
−30
140
100
PS
−10
60/80
50/70
SANa
−20
95
85
SBa
−20
60/80
50/70
PVC (hard)
−5
75/100
65/85
PVC + softener
0/−20
55/65
50/55
Table 24.2
Chemical resistance of some plastics
HDPE | LDPE | PET | PP | COC | PS | SAN | SB | PVC | Soft-PVC | |
|---|---|---|---|---|---|---|---|---|---|---|
Water cold | + | + | + | + | + | + | + | + | + | + |
Water warm | + | ? | ± | + | + | ± | + | ± | ± | ± |
Acids weak | + | + | + | + | + | + | + | + | + | + |
Acids strong | + | ± | ± | ± | + | ± | ± | ± | + | − |
Acids oxidising | − | − | ± | − | + | − | − | − | ± | − |
Base weak | + | + | ± | + | + | + | + | + | + | + |
Base strong | + | + | − | + | + | + | + | + | + | − |
Sol.anorg.salt | + | + | + | + | + | + | + | + | + | + |
Halogens | − | − | − | ± | − | − | ± | − | ± | − |
Aliphatic hca | + | + | − | + | − | − | + | ± | + | − |
Choride hca | ± | − | ± | − | − | − | − | − | − | − |
Unsat.chlor.hca | − | − | ± | − | − | − | − | − | − | − |
Aromatic hca | ± | − | − | ± | − | − | ± | − | − | − |
Alcohols | + | ± | − | + | + | + | + | − | + | − |
Esters | + | ± | ± | ± | ± | − | − | − | − | − |
Ketones | + | ± | − | ± | ± | − | − | − | − | − |
Aldehydes | ± | ? | ? | + | ± | − | − | − | − | + |
Ether | ± | − | ± | + | − | − | − | − | − | − |
Amines | + | ? | ? | + | ? | + | + | + | ± | + |
Organic acids | + | + | + | ± | ± | ± | ± | ± | ± | ± |
Mineral oils | + | ± | + | + | − | + | + | ± | + | ± |
Lipids, oils | + | ± | + | + | − | − | − | − | + | ± |
Table 24.3
Other characteristics of some plastics and glass
PVC | PVC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
HDPE | LDPE | PET | PP | COC | PS | SAN | SB | Hard | Soft | Glass | |
Impact resistance | ± | + | + | ± | − | − | − | ± | ± | + | − |
Flexible | − | + | − | − | − | − | − | − | − | + | − |
Transparent | − | + | + | ± | + | + | + | ± | + | ± | + |
Colouring | + | + | + | + | + | + | + | + | + | + | + |
Permeable to gas | − | ± | ± | ± | − | + | + | + | ± | ± | − |
Biocompatible | + | ± | + | ± | + | − | ± | − | ± | − | + |
Permeable to liquid | − | ± | ± | ± | − | + | + | + | ± | ± | − |
Leaching of additives | ± | − | − | ± | − | − | − | ± | ± | + | − |
Resistant to 15 min at 121 °C | + | − | − | + | + | − | − | − | − | * | + |
Streaming water vapour | + | − | − | + | + | − | +? | − | * | * | + |
During the production process of a plastic, additives can be added to:
Affect the production process, for example catalysts
Improve polymer stability, for example antioxidants
Alter the mechanical characteristics, for example plasticisers
Improve the visual appearance, for example colorants
Ph. Eur. specifies a number of additives allowed for plastics for containers for pharmaceutical products [7]. Because of the addition of additives, plastics from different suppliers do not have the same composition. Always request the composition of the plastic container being purchased, from the supplier, and only change the containers of one supplier for those from another after considering the differences in the plastic. A raw material or a preparation cannot just be repacked from a certain plastic container to a container from another plastic without reassessing the shelf life. For authorisation of medicines an extensive declaration of the composition of the primary plastic container is demanded and declarations of technical characteristics, suitability and toxicity have to be submitted.
The characteristics and use of the plastics will be discussed one by one.
24.2.3.1 Polyamide (PA)
Polyamide is a polymer formed from condensation of dicarbonic acids and diamines or from condensation of amino acids and lactames. In publications, in English, polyamids are called nylon. To distinguish them from one another a number is added that represents the number of C-atoms of the monomer or monomers. Nylon type 6.6 (polyamide 6.6) is the most common commercial grade of nylon. It consists of hexamethylene diamine and adipinic acid.
The characteristics of polyamides depend very much on the composition. They are generally wear-resistant and all forms are hygroscopic. This absorption of water (plastifying effect) significantly changes the properties of polyamides. The thermal resistance of most polyamides is high. They can be sterilised by steam or gamma radiation; polyamide 6.6 can even be sterilised by hot air.
24.2.3.2 Polycarbonate (PC)
The most common polycarbonate is a polymer generated from condensation of bis-phenol-A (= 2,2-diphenylolpropane). Polycarbonate has good thermal and mechanical characteristics. It is a stiff material which is fraction- and impact-resistant and also resistant to strongly diverse temperatures.
Polycarbonate is not resistant to alkali, strong acids and organic solvents (for example chloroform and acetone). The gas permeability of polycarbonate is relatively high compared to for example polyethylene, and comparable to polystyrene.
24.2.3.3 Polyethylene (PE)
Polyethylene (= polyethene = polythene) is a polymer that consists of ethylene units. The different forms are determined by the method of production:
Low density – polyethylene (LDPE)
High density – polyethylene (HDPE)
LDPE is produced under high pressure (1,500 bar) and temperature (150–240 °C), in the presence of catalysts. HDPE is produced under low pressure (0–2 bar) and temperature (70 °C), in the presence of catalysts. HDPE is a stiff type of polyethylene whilst LDPE is more flexible. An increase of the density gives a reduction of the flexibility and a not fully transparent product. However, other characteristics are improved by increasing the density.
For HDPE compared to LDPE:
The permeability to water vapour is lower
The permeability to gas is lower
The chemical resistance is higher
The light transmission is lower
The temperature resistance is higher
Both HDPE and LDPE are plastics that are widely used because of their low permeability to water vapour and their high chemical resistance. PE is very resistant to gamma radiation.
24.2.3.4 Cyclic Olefin Copolymer (COC)
Cyclic olefin copolymers are a new class of polymeric materials based on cyclic olefin monomers (as 8,9,10-trinorborn-2-ene) and ethene. These materials are also known as cyclic olefin polymers (COP) when only one single type of cyclic olefin monomer is applied. COC is very transparent, the optical properties are in many ways similar to glass. COC is one of the few transparent polymeric materials able to withstand steam sterilisation. Permeability to water vapour and gas is low. COC shows good chemical resistance to alcohols, acids and bases, but it is attacked by non-polar solvents.
COC vials or syringes can be an alternative to glass containers. Strong points are good barrier properties, good chemical resistance, high purity and low leachables of the material, its clarity, and compatibility with sterilisation by gamma radiation, steam, or ethylene oxide. Being a stiff polymer material, impact resistance can be low compared to more flexible materials.
24.2.3.5 Polyethylene Terephthalate (PET)
Polyethylene terephthalate is a polyester. It is a linear ester composed of ethylene glycol and terephthalic acid. PET has excellent mechanical characteristics; it is for example pressure- resistant. The chemical resistance is good compared to other plastics. PET is not resistant to strong alkali and limited resistant to strong acids and chlorinated hydrocarbons (see Table 24.2).
24.2.3.6 Polypropylene (PP)
Polypropylene (= polypropene) generates from polymerisation of propene. With stereo specific catalysts three types of polypropylene can be produced:
Syndiotactic polypropylene; the methyl groups are alternately positioned with respect to the polymer chain.
Isotactic polypropylene; all methyl groups are placed on the same side of the polymer chain.
Atactic polypropylene; the methyl groups are randomly placed with respect to the polymer chain.
The position of the methyl groups defines the extent of crystallinity and therefore the mechanical and chemical behaviour. Isotactic polypropylene for example is stiffer, harder and stronger than atactic polypropylene. The polypropylene that is used for containers usually consists of a high percentage of isotactic polypropylene. Atactic polypropylene is not used pharmaceutically.
Both chemical and physical characteristics of polypropylene resemble high density polyethylene, but polypropylene is clearer, harder, has a lower density and a better thermal resistance (polypropylene can be steam sterilised). Polypropylene is less resistant to low temperatures than high density polyethylene. The chemical resistance of polypropylene is good (see Table 24.2). To protect polypropylene from oxidation, antioxidants are always added. Resistance to gamma sterilisation is relatively poor, but can be enhanced by additives.
24.2.3.7 Polystyrene (PS)
Polystyrene is generated from the monomer styrene by polymerisation in the presence of a catalyst. It is a crystal-clear plastic, easy to colour and completely free of odour and taste. Polystyrene is quite hard and brittle (little impact-resistance). It is resistant to acids, alkali, alcohol and inorganics. It is not resistant to boiling water and many organic solvents, such as chloroform and ether, or to fatty oils. Therefore polystyrenes are not suitable for packaging dermatological products containing fats.
The characteristics of polystyrene can be modified by:
Copolymerisation of styrene with butadiene (SB). This generates an impact-resistant polystyrene. This modification however results in a loss of transparency and necessitates the addition of stabilizers such as antioxidants.
Copolymerisation of styrene with acrylonitrile (SAN). This generates a transparent polystyrene that has a better resistance to chemicals and higher temperatures.
The permeability to oxygen and water vapour of polystyrene and modified polystyrene is relatively high.
24.2.3.8 Polyurethane (PUR)
Polyurethane is a collective term for a group of polymers that contain an urethane group (-NH-COO-). They are generated from a reaction between polyesters and polyethers with di- or poly-isocyanates.
Dependent on the starting materials, polymers with strongly diverse characteristics can be produced, varying from thermoplasts to thermosets.
Polyurethane has a range of packaging functions and is often used as filling material in tablet containers. Polyurethanes do not have a good resistance to acids and alkali.
24.2.3.9 Polyvinylchloride (PVC)
Polyvinylchloride is used as basic material for the production of infusion bags. It can also be found in blister packages. Polyvinylchloride is generated from the monomer vinylchloride with the help of a catalyst. Pure PVC is a hard, transparent plastic.
Additives in PVC for infusion bags are: stabilisers and plasticisers. By adding a plasticiser a flexible, soft plastic is formed. The amount of plasticiser in PVC can rise to 60 % of the total weight. Plasticisers that are often used are di(2-ethylhexyl)phthalate (DEHP) and dioctylphthalate (DOP). DEHP is the only softener that the Ph. Eur. allows to be used in PVC as material in containers for blood, blood products and aqueous solutions that are used for intravenous infusion. In this case the amount of DEHP in PVC should not be more than 40 % [7].
DEHP is a lipophilic compound and can therefore migrate from the PVC into medicines that contain lipophilic or adsorbing substances. Exposure of the patient to softeners should be minimized. Zinc or calcium salts are used as stabilizers. The use of stabilizers is also limited by the Ph. Eur. [7].
The interaction between PVC and liquid medicines has been studied extensively [20].
Critical topics include the release of softener from the PVC as well as the sorption of medicines. Sorption of medicines occurs more to PVC than to glass and polyethylene [21].
PVC can be sterilised in a steam steriliser with pressure compensation.
Hard PVC is used for blister packages. It is preheated in a blistering machine and then round or capsule-like cavities are being formed in a moulding station. PVC is relatively permeable to water vapour and therefore not suitable to contain hygroscopic tablets or capsules. However, water permeability is greatly reduced when the PVC-film is combined with a thin layer of PVDC (polyvinylidene chloride).
24.2.4 Rubber
Rubber is the starting material of elastic packaging materials that are used in closures of containers. Natural rubber, bromobutyl rubber, chlorobutyl rubber and silicone rubber can be distinguished [22–24].
Rubbers are generated by vulcanisation (crosslinking) of elastomers. Elastomers are macromolecular organic compounds that are formed from natural or synthetic substances. In the production process additives are being used as vulcanisers, catalysts, stabilizers, colourings, fillers and lubricants, see Table 24.4.
Table 24.4
Composition of rubber
Component | Weight percentages |
|---|---|
Polymer (elastomer) | 40–95 |
Vulcanisers | 1–4 |
Catalyst | 2–8 |
Filler | 0–60 |
Colouring | 0–10 |
Lubricant | 0–4 |
Stabilizer | 0–3 |
The different rubbers distinguish themselves by three factors:
The basic elastomer
The method of vulcanisation
The amount and type of additives
The choice of the rubber depends on the preparation and on the functionality of the container:
Adsorption from components of the preparation to the surface of the rubber as well as migration into or through the rubber should not happen.
The rubber should not release substances into the preparation.
The material should be suitable for its use; rubber closures that are being used for multiple piercing by needles have different requirements than the dropper of an eye drop bottle.
Which rubber is selected for use in a particular pharmaceutical container not only depends on the compatibilities or the most suitable physical characteristics, but also on the availability. In actual practice only industrial producers of medicines are able to choose the most optimal container.
Table 24.5 gives an overview of the characteristics of some rubbers.
Table 24.5
Characteristics of some rubbers
Characteristic | Natural rubber | (Halogen-) Butylrubber | Silicone rubber | Ethylene-Propylene rubber |
|---|---|---|---|---|
Elasticity | Very good | Moderate | Good | Good |
Deformation rest | Very little | Moderate/large | Very little | Little |
Permeability (gas and water vapour) | Very high | Very little | Very high | Moderate/high |
Reactivity (ageing) | High | Very little | Very little | Very little |
Temperature resistance | Very poor | Very good | Very good | Very good |
Release of substances | Possible | (Less) possible | Less possible | Possible |
24.2.4.1 Vulcanisation Methods
There are three vulcanisation methods: with sulfur, with bifunctional reagents and with peroxides. Most importantly, the vulcanisation method determines which contaminations will be present in the product. The vulcanisation density is the number of bonds between different chains per volume-unit.
Vulcanisation with sulfur
Elementary sulfur or compounds that can be used as a source of sulfur form together with suitable additives at higher temperatures thio-ether-, disulfide- or polysulfide-bridges in and between chains. This vulcanisation method is primarily suitable for those elastomers that have unsaturated bonds. The rubber produced by this method has good mechanical characteristics. However, a disadvantageous chemical characteristic of rubber vulcanised with sulfur is that additives can leach into the product. An example is the release of thiol compounds, which are incompatible with some mercury compounds.
Vulcanisation with bifunctional reagents
The reagent, for example a diamine, forms covalent bonds with the polymer chains. Usually the use of catalysts is not necessary. This vulcanisation method is mainly used for the production of halogen butyl rubbers and results in materials with good chemical characteristics (few incompatibilities).
Vulcanisation with peroxides
The peroxide serves as a producer of radicals and is not being built into the polymer. The result of this vulcanisation is a chemically inert product. The use of a catalyst is not necessary and with a right choice of the peroxide, combined with an after-treatment of the material (extraction of the peroxide) a rubber that releases only very small amounts of substances is produced. This method is being used for the production of silicon-elastomer and of ethylene-propylene rubber.
24.2.4.2 Additives
Silicates, silicic acid, calcium carbonate or barium sulfate are used as fillers. Silicates and silicic acid make rubber more sustainable. Colourings for rubber are for example titanium dioxide and iron oxides. It is better to avoid organic colourings due to the chance of migration of the colouring to the rubber surface, followed by migration into the preparation.
24.2.4.3 Natural Rubber
The oldest form of rubber is natural rubber. The basic elastomer of natural rubber is taken from the latex of the rubber tree (Hevea brasiliensis, Euphorbiacea family). Latex consists of carbohydrates, water, fatty acids, proteins and stearines. The complex composition of latex varies significantly with origin, season, etc. This variation in composition is the main disadvantage when using this material in pharmaceutical products.
Natural rubber consists of polyterpenes. The gross formula of this basic elastomer in natural rubber is (C5H8)n. The module is isoprene (the base of all terpenoides). It is a polyunsaturated polymer with a structure as shown in Fig. 24.1. The value of n is very high; the molecular weight lies between 200.000 and 300.000.
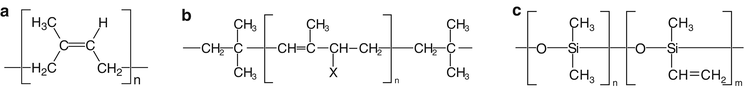

Fig. 24.1
Structural formulas of (a): basic elastomer in latex; (b): basic elastomer of butyl rubber, in which x = H for butyl rubber; x = Cl for chlorobutyl rubber; x = Br for bromobutyl rubber; (c): basic elastomer of silicone rubber
The chemical structure of the natural latex product determines the characteristics of the rubber produced from that latex:
Natural rubber can, dependent on composition and origin, release foreign substances (for example UV-absorbing) to a preparation.
Due to high unsaturation of the basic elastomer, a high crosslink or vulcanisation density can be generated. This results in good mechanical characteristics, as a highly elastic product is generated.
Due to the high number of unsaturated bonds that are still present in the end product after vulcanisation, the natural rubber is a chemically reactive compound. This reactivity significantly reduces the chemical compatibility and useful shelf life of natural rubber.
Due to its structure, natural rubber has a high permeability to gas and water vapour.
Natural rubber is susceptible to the absorption of many substances (for example preservatives such as chlorobutanol and phenylmercuric borate).
24.2.4.4 Butyl Rubber, Bromobutyl Rubber and Chlorobutyl Rubber
The basic elastomer of butyl rubber is the synthetized polymer poly-isobutylene with approximately 3 mol% isoprene. In case of the halogen butyl rubbers the isoprene is replaced by halogen isoprene. The structure of the basic elastomer is shown in Fig. 24.1. The basic elastomer of butyl rubber contains relatively few unsaturated bonds compared to the basic elastomer of natural rubber. The reactivity of butyl rubber is therefore less than that of natural rubber. To advance the vulcanization process, a higher concentration of reagents and/or a longer reaction time and a higher reaction temperature are therefore necessary. This relatively high amount of additives in butyl rubber can cause problems when it comes into contact with the pharmaceutical preparations. ‘Leaching’ may occur, where dispersed (not chemically bound) additives migrate to the surface of the rubber, followed by extraction into the preparation.
When producing the bromo- and chlorobutyl rubbers, the halogen basic elastomer is used. Like the non-halogen basic elastomer, this contains relatively few unsaturated bonds. The halogen atom activates the double bond and increases the reactivity of the basic elastomer. Therefore no extra measures to accelerate the vulcanization process have to be taken, unlike the production of butylrubber. The halogen butylrubbers therefore have largely replaced butylrubber.
The chemical structure of the halogen basic elastomer determines the characteristics of the halogen rubber which is synthesised from this basic elastomer:
Due to the fact that the basic elastomer is mainly unsaturated, only a small amount of crosslinking- or vulcanisation density is generated. This leads to a product with relatively modest mechanical properties, when compared to natural rubber.
Due to low levels of unsaturated bonds in the final product after vulcanisation, the halogen butyl rubber is chemically unreactive; hence the product is inert and has a long shelf life.
During the production process of halogen butyl rubbers relatively few additives are necessary and the chance that additives are leached from the rubber into the pharmaceutical preparation is less when compared to natural rubber or butyl rubber.
Permeability to gasses and water vapour is much less for halogen butyl rubbers than for natural rubber.
Summarising: the mechanical characteristics of halogen butyl rubbers are not optimal (stronger deformation, less elastic than natural rubber), but the saturated structure leads to excellent chemical characteristics (see Table 24.5).
Rubber stoppers to close injection- or infusion bottles and –bags can be coated with a thin layer of a polymer that resembles Teflon (Teflon = polytetrafluorethene, PTFE). The result is a chemically inert stopper with significantly less “leaching” of substances from the rubber.
24.2.4.5 Silicone Rubber
The basic elastomer for silicone rubber is a linear polysiloxane, built from dimethyl siloxane units with a small amount methylvinyl siloxane groups. The ends of the chains are formed from trimethyl siloxane- or dimethylvinyl siloxane groups. The structure of the basic elastomer is shown in Fig. 24.1. Peroxides are usually used for the crosslinking of silicone rubbers (for example dicumyl peroxide or 2,4-dichlorobenzoyl peroxide).
To improve tear resistance, the additive silicic acid can be added. Other additives are usually not necessary, so migration of substances from the final product into the pharmaceutical preparation will usually be limited to residues of the peroxide. By the right choice of the peroxide and adequate after treatment of the final product this problem can be reduced. The Ph. Eur. contains a test for the presence of peroxide residues in the elastomer.
Silicone rubber, like other rubbers that are produced following the peroxide method, can give an aldehyde odour shortly after production. This is caused by traces of peroxide breakdown products. The odour disappears with time or after steam sterilisation.
Silicone rubber is very resistant to ageing and oxidation. It shows a very high permeability to gas and water vapour, which significantly reduces the pharmaceutical usefulness. Silicone rubber is very resistant to a wide range of chemicals. It is resistant to weak acids and alkali, salt solutions and mono- or multivalent alcohols and phenols. However, it is not resistant to strong acids and bases. In low molecular solvents (ketones, esters, aliphatic, aromatic and chlorinated hydrocarbons) a reversible swelling of the elastomer occurs. Many preservatives and active substances are strongly adsorbed into silicone rubber.
The mechanical characteristics of silicone rubber are very highly independent of temperature. The elastomer keeps its original hardness and elasticity with temperatures lower than -50 °C. Resistance to high temperatures is also very good (to 250 °C). Addition of metal oxides to silicone rubber can improve the temperature resistance even further.
24.2.4.6 Ethylene Propylene Rubber
The basic elastomer ethylene propylene monomer (EPM) is generated by copolymerisation of ethylene and propylene. As the polymer chain does not have double bonds, vulcanisation takes place with the use of organic peroxides. The ethylene propylene rubber that is thus generated contains no double bonds. Therefore, the product is inert and has a long shelf life.
Ethylene propylene diene polymers (EPDM) are basic elastomers with double bonds in the side chain. They are generated by adding small amounts of diene monomers in the copolymerisation of ethylene and propylene. Due to the presence of this unsaturated bond in the basic elastomer both vulcanisation with peroxides and vulcanisation with sulfur are possible. Vulcanisation with peroxides is usually chosen. This generates a product that is relatively inert and well resistant to ageing.
When synthesising ethylene propylene rubber usually a filler (for example aluminium silicate or calcium carbonate) and a plasticiser (for example an aromatic hydrocarbon) are added and a colouring if necessary.
As with silicone rubber, ethylene propylene rubber can give an aldehyde odour. Ethylene propylene rubber is highly resistant to water, alkali and dilute acids, and moderately to poorly resistant to hydrocarbons, lipids and concentrated acids. It is resistant to the high temperatures of steam sterilisation.
24.2.5 Paper and Cardboard
Paper is a single-layer material (7–250 g/m2); cardboard is a multiple-layer material (250–600 g/m2). Both are made from cellulose fibres that are compressed.
Paper is the primary packaging layer for the packaging of single dose oral powders in powder paper (Sect. 24.4.12). However, it is more common for paper and cardboard to be used as secondary or tertiary packaging layer. Paper is also the basic material of a special packaging material: the label (Sect. 24.2.6).
Powder paper should not be contaminated, should not shed fibres and within one batch there should not be much difference in weight per surface area (= constant powder paper weight).
For weighing of fatty or semisolid starting materials so called greaseproof paper can be used. This type of paper is smoother and stronger compared to ‘normal’ powder paper. Lipid penetrates slower into the paper, but interactions (migration) are not excluded.
Cardboard is a popular material for the secondary container. It is relatively cheap, easy to design in suitable shapes and easily printed with information. Cardboard is often used as tertiary container to protect the packaged products during transport. The objectives determine the requirements that are set for the material. For example, to send a package to the tropics, a cardboard box with an aluminium inside layer is appropriate to protect the contents against moisture.
24.2.6 Labels
The label is an important part of the pharmaceutical product. The most common label used within the European pharmacy is the self-adhesive label. This consists of a laminate of two special types of paper: the carrier material and the label paper. The carrier material consists of paper on which a silicon layer is fixed. The label paper can be printed on the printing side and on the carrier side it has an adhesive layer. The adhesive is usually of the permanent type, rendering the label non-removable once applied [25]. Label adhesives on plastic containers such as infusion bags require additional attention, see Sect. 24.4.13.
Uniform labels of good constant quality are very important for pharmacy preparation. Quality control of incoming labels, both printed or unprinted, should equal the requirements for containers described in Sect. 24.5.
The way in which information should be put on labels (labelling) is dealt with in Sect. 37.3.
24.3 Closures
24.3.1 Closure Systems and Functions
The container closure system seals the primary container after filling, protecting the contents from interactions with the external environment. In the majority of cases, the closure also allows access and use of the product. Where tamper evidence is required the closure system must be able to show that the container has been opened.
In order to provide these functions, the forms and methods used to provide an effective seal vary widely. The closure systems used in pharmacy usually depend on a system were a relatively soft material is pressed onto a hard material. The softer, more resilient material will compensate for small imperfections on the hard surface, leading to an effective seal. The materials forming the closure are pressed together by means of:
Threaded screw cap. An example is the bottle screw cap with a relatively soft liner material on the inside of the cap. A screw cap made of (softer) thermoplastic materials often doesn’t need an additional liner material. The closing torque force must be sufficient to provide a good seal.
Material deformation. An example is the vial closure for injectable drugs. The glass container is sealed by an elastomeric stopper, firmly locked in place by means of an aluminium overcap that is mechanically crimped over the stopper and neck of the vial.
A snap-on closure where a raised ring is forced over a bead or lip. An example is the Sterillab snap cap eye drop bottle (Sect. 24.4.2).
A push-in closure where a tight-fitting stopper is pushed in place and stays closed by friction forces.
The other preferred form of closure system in pharmacy is heat-sealing. This usually achieves an effective and permanent seal, which can only be opened by destruction of part of the packaging material. An example is the glass ampoule, fused by heat. The fused glass forms a totally hermetic seal. Another example is the blister packaging of tablets. A multi-layered film of polymer material is preformed and filled with tablets, and sealed by heat and pressure to a thin aluminium strip foil.
24.3.2 Container Closure Testing
The design and manufacture of quality packaging materials is a science in itself. Container closure quality is essential and needs to be demonstrated as an integral part of the design of the medicinal product. This task can be most challenging when thousands of containers need to be reliably filled and closed on pharmaceutical packaging lines.
Many different procedures have been developed to test container closure quality:
1.
Dye ingress test: the closed container is submerged in a dye solution. Leaks are more easily detected by the application of external (air) pressure and the addition of a wetting agent to the dye.
2.
Air leak detection: the closed container is submerged under water and a vacuum is applied. The addition of a wetting agent helps in the detection of air leaks.
3.
Liquid loss: the filled and closed container is inverted and checked for liquid leaks. Application of a vacuum helps to identify a poor seal.
4.
Water vapour permeability: a container is filled with water, closed and stored in a warm and dry environment. Weight loss by water evaporation is followed. Alternatively, a desiccant is placed inside the container and the closed container is stored in a high relative humidity environment. Weight gain through moisture is followed. This last test is suitable to test tablet blister packaging and is the preferred method in USP <671 > Containers – Performance Testing.
5.
Oxygen permeability: a solution of easily oxidisable drug (ascorbic acid, or acetylcysteine) is filled in the container and the container is closed using nitrogen gas flushing to exclude any oxygen in the head space. At suitable time points, containers are sampled and the ingress of oxygen can be followed by quantification of drug loss.
The above list is not comprehensive and usually performance testing using selected tests will be sufficient. Many variations or combinations of these test methods can be made to tailor a particular packaging system. Testing is performed as part of the product development phase and can be a part of container and closure quality testing on incoming materials, or even production process monitoring. In small-scale production, it is recommended to request from the vendor any known data on container closure performance testing as a container system quality attribute. For any pharmaceutical application, it is wise to perform in-house testing of the container closure to validate the outcome of the packaging process. All that is needed is a balance and if possible some desiccant.
24.4 Packaging Forms
In this section the different types of containers for pharmaceutical preparations will be discussed. Definitions, terminology, functional requirements and materials used for the containers for pharmacy preparations will be dealt with.
Definitions and terminology are taken from the list of standard definitions of the European Directorate for the Quality of Medicines (EDQM) [26].
Besides discussing packaging forms such as bottles, tubes, strips and bags some generally used dosage delivery devices (Sect. 24.4.19), child-resistant containers (Sect. 24.4.20), containers for arthritic patients (Sect. 24.4.21) and stock containers (Sect. 24.4.18) are also discussed. Delivery devices that are specific for a special administration route will be discussed in the chapters on dosage routes and forms.
24.4.1 Bottles
A bottle is a “container with a more or less pronounced neck and usually a flat bottom” [26].
Bottles may be used to package liquid preparations for a variety of administration routes:
Oral liquid preparations: oral solutions, suspensions and emulsions, drops for oral use
Rectal preparations: enemas
Dermal preparations: solutions, suspensions and emulsions for cutaneous use, shampoos
Parenteral preparations: injection solutions, infusion solutions
Preparations for the eye: eye drops, eye lotions
Solutions for irrigation of the bladder, the vagina, for wounds
Preparations for throat, nose and ear: mouth washes, gargles, ear drops, nose drops, nasal sprays
Various: stock containers for base solutions for example
Different administration routes lead to different requirements and different designs of bottles. These bottles will also require different closures and different delivery devices. This section describes the general characteristics of these bottles and the materials used to manufacture them. In Sects. 24.4.2, 24.4.3, 24.4.4, 24.4.5 and 24.4.6 some specific bottles are discussed. In Sect. 24.4.19 closures and delivery devices are discussed.
24.4.1.1 Requirements
In addition to the general requirements for product protection (Sect. 24.1.1), handling and carrying information (Sect. 24.1.2) there are several supplementary requirements for bottles:
A similar filling opening for all volumes
Standardized dimensions for the neck of the bottle to enable the use of standard closures and dosage delivery devices (see Sect. 24.4.19)
A grade mark on the bottle to indicate the fill volume
For glass bottles the Ph. Eur. describes general specifications. The suitability of the container for the preparation should always be assessed. Type I glass (see Sect. 24.2.1) is considered to be suitable by the Ph. Eur. for all preparations. Type II glass is not suitable for aqueous parenteral products with pH >7. Type III glass is unsuitable for aqueous parenteral products. These specifications are based on the potential generation of particles in the solution (see Sect. 24.2.1). These guidelines will also apply to other pharmaceutical products which are meant to be free from particles (for example eye lotions).
24.4.1.2 Materials
Materials generally used for bottles are:
Glass, transparent or amber; required hydrolytic resistance depends on usage
Plastic; mainly PET (polyethylene terephthalate), PP (polypropylene) and sometimes PE (polyethylene)
The most significant advantages of plastic over glass are low weight and high fracture resistance. A major disadvantage is the possible deformation when heated.
Glass and plastic differ in permeability and interactions between the packaging material and contents. Glass is the least permeable to air (oxygen) and liquid. The permeability increases in the sequence: glass < HDPE < PP < PET. Glass, PP and PET generally present few interaction problems; HDPE can show adsorption. More material characteristics are described in Sect. 24.2.
Table 24.6 provides an overview of the materials that are being used for packaging different preparations in bottles.
Table 24.6
Materials of bottles, jars and bags for different pharmacy preparations
Preparation | Glass | Plastic | |||||
|---|---|---|---|---|---|---|---|
I | II | III | PE | PP | PVC | PET | |
Oral | |||||||
Liquid | + | + | + | + | + | ||
Solid | +
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|