Fig. 37.1
Standardised label for Lugol’s Solution FGP (From Formulário Galénico Português with permission)
The legal rules for specific labelling for ambulant patients in most countries do not apply to hospitalised patients. But many hospital pharmacies will have their own guidelines, normally including the following minimum requirements for labelling for an individual patient:
Name of active substance, form and strength and quantity issued
Essential warnings i.e. do not crush or chew; shake the bottle
Patient’s name (and sometimes a hospital identification number)
Date
Ward that the patient is on
In some countries professional standards are in force. In these countries guidelines for the labelling of medicines in the hospital have been published.
In other countries working groups of hospital pharmacists are preparing such guidelines for special groups of medicines, i.e. antineoplastics or paediatric medicines.
37.3.2 Labelling and Package Leaflet in More Detail
Labelling should fulfil the legal requirements, preferably in a way that helps the patient. In some countries, organisations of, for example, hospital pharmacists may have developed their own guidelines on labelling. This section comments on how to deal with the legal requirements, more or less in the order they appear on the label. It is important to note that legislation at this point varies from one country to another. As a consequence this chapter can only give general recommendations.
37.3.2.1 Route of Administration
The uppermost zone of the label shows the route of administration, if this not the oral one. Officially the Standard Terms of the European Directorate for the Quality of Medicines and Health Care (EDQM) [6] should be used, but in some countries all non-oral routes may be indicated as ‘Not to be taken’. A more specific term such as Eye drops, Ear drops or ‘For rectal use’ is to be preferred. Some patients may not know the meaning of the term ‘rectal use’. Also the words ‘Not to be taken’ sometimes need explanation, for instance in the case of a mouth rinse.
The use of colours, either for the complete label or for a strip at the top to emphasise non-oral use is widespread, and in some countries compulsory. The colour may differ per country. A blue strip is often used, but a red label is common in other countries, e.g. Croatia and the Czech Republic. Also other colours on the label may be used as a signal for certain groups of medicines, e.g. in hospitals.
37.3.2.2 Name of the Pharmacist or Pharmacy
Although legislation will vary, name, and address or telephone number of the dispensing pharmacy should always be clearly indicated. Sometimes the legislation specifies that the name of the owner or the pharmacist should be on the label, or other ways of identification of the pharmacy are used.
The Pharmacy Practice Order (Apothekenbetriebsordnung) in Germany demands, besides the name of the owner, the initials of the person who has dispensed the medicine on the label, or the name of the supervising pharmacist [7]. In Croatia and the Czech Republic this applies for the label of pharmacy preparations. In the UK it is good practice to write the initials of the pharmacist and those of the dispenser if they are different, but this is not law.
37.3.2.3 Date
Usually the date the medicine is dispensed will appear on the label. It is important to notice that this date may be different from the date it is actually delivered to the patient. The date can be used as an instrument for tracing a dispensed medicine, but in most of the national legislation a batch number is required for pharmacy preparations
37.3.2.4 Name of the Patient
Just the name is usually not enough to identify a patient. Date of birth, first name(s) and address give additional information. Hospitals may use a unique identification number. In delivering a medicine to someone else than the patient in person, the pharmacist assumes responsibility. When in doubt, inquiries from the patient himself, or a proof of identity may be asked.
37.3.2.5 Name and Strength of the Medicine
For the indication of the active substance in the name of pharmacy preparations the generic or International Non Proprietary Name (INN) is to be preferred. The purpose of the strength in the name is to indicate the quantity of the active substance, which is relevant for the correct use and identification of the product, and its distinction from similar presentations. The strength in the name should, therefore, be based on user/prescription criteria rather than analytical criteria according to the Recommendations on the expression of strength in the name of centrally licensed human medicines from the Quality Review of Documents group (QRD) [8].
At the same time it should be clear which form of the active substance is meant in the indication of the strength. This can for instance be the hydrate of a salt, or just the active moiety, without the salt or hydrate water (see Sect. 23.1).
For instance, in morphine preparations normally morphine hydrochloride trihydrate or morphine sulphate pentahydrate will be used as raw material. These salt hydrates are included in the Ph.Eur. and BP (British Pharmacopoeia) respectively [9]. Then if the name would say just morphine, the strength should refer to the morphine base moiety. But as usual doses refer to the salts, the salts should be indicated in the name. Examples are:
Morphine sulphate injection 10 mg/ml BP
Morfinehydrochloridedrank (Morphine Hydrochloride Oral Solution) 20 mg/mL FNA
The same applies to many generic medicines, e.g. Metformin hydrochloride 1000 mg tablets. These could also be called: Metformin 780 mg tablets (as hydrochloride), but this would be very confusing, as dosing regimens always use the hydrochloride.
For inorganic compounds both cation and anion are always mentioned. Abbreviations for the elements are allowed. The EMA now recommends that name and strength of licensed medicines should refer to the active substance or moiety [1]. Therefore in preparations that were licensed by EMA recently, the name and dosing schemes usually refer to the active substance only, even if it is present in a salt form. For instance Pradaxa® 75 mg capsules contain dabigatran etexilate mesilate, equivalent to 75 mg dabigatran etexilate.
Sometimes the strength is indicated in two ways. For instance in Sifrol® 0.7 mg each tablet contains 1.0 mg pramipexole dihydrochloride monohydrate equivalent to 0.7 mg pramipexole. Because doses, as published in the literature, refer to the salt form, the name on the package is Sifrol® 1 mg (0.7 mg base).
37.3.2.6 Ingredients of the Medicine
In some countries it is permitted to use, for preparations included in a national formulary, the product name mentioned in that formulary, provided the formulation corresponds exactly with the one in the formulary (e.g. Hydrocortisonacetaatcrème FNA in the Netherlands). Adding the name of the formulary is necessary in that case to identify the formulation. Additional information about the ingredients should be in the package leaflet. For cutaneous preparations in particular it is important that prescribers can obtain this type of information. Special editions of a national formulary, intended for prescribers, exist in some countries.
If the product is not a standard formula, the names of all the active ingredients have to appear on the label, and preferably of all the excipients as well. At least preservatives and antioxidants should be mentioned. The EMA requires excipients known to have a recognised action or effect to be mentioned [1]. A list of such excipients is available on the EMA website [10]. Examples are ethanol and propylene glycol, and benzyl alcohol, especially in medicines for children. See also Sect. 5.4.5.
In naming pharmacy preparations with more than one active substance, it will in practice not always be possible to follow the rules about the name and the strength. If for instance 0.1 % triamcinolone acetonide is added to ketoconazole cream, the name on the label for the patient would probably be: 0.1 % triamcinolone in ketoconazole cream, simply because the full name would not fit.
Therefore it is important to list the full formula on a separate label.
Names of substances should be written in full, including water of hydration, if appropriate. For substances of pharmacopoeia quality the name as mentioned in the Ph. Eur. may be used, followed by the initials, Ph. Eur. In this notation it is usually not necessary to state the amount of water of hydration, as this will be defined in the Ph. Eur. monograph. Using the Latin name of the Ph. Eur. for the substance may be an alternative way to indicate their quality.
37.3.2.7 Content of Active Substance in Pharmaceutical Preparations
The amount of active substance should be declared as ‘pure substance’. This means the content that would be determined in a chemical assay. For example, in the preparation a calculated excess of active substance is processed, because the material may contain water of hydration. This excess should not appear on the label (i.e. the label claim), as the assay refers to the active substance without water.
However, when the quantity of active substance refers to a hydrate, this should appear on the label. The content of the hydrate should then be determined.
For preparations presented in single-dose-units it is important to indicate the composition per dosage unit, as well as the number of units supplied. As a consequence it will sometimes be necessary to calculate the quantity of excipients per unit from the amount used for the whole batch (i.e. fillers in capsules). When only the quantities of ingredients for the whole batch are indicated in the label, there is the risk that other caregivers will interpret the composition falsely. For the same reason, according to the EMA the concentration of oral liquids and other multidose forms should be indicated per millilitre or per dose, so not as a percentage [8]. For semisolid preparations like creams and ointments, the concentration or strength should be indicated as amount per unit weight (e.g. mg/g).
37.3.2.8 Units
For the indication of strength or amounts of active substances and excipients the following physical parameters are used: volume, mass and or quantity, with units according to the international system (SI). The following units and derived units are used:
Volume: litre (l), millilitre (ml); however according to the Ph. Eur. the litre is written with a capital L: litre (L) and millilitre (mL); mass: kilogram (kg), gram (g), milligram (mg) and microgram (microgram rather than μ, μg and mcg)
Quantity: gram molecule (mol), milligram molecule (mmol), microgram molecule (micromol, rather than μmol or mcmol)
Despite of the fact that μg is the SI unit, the use of this abbreviation should be discouraged. Some fatal errors have occurred in the past, as a result of false readings of drug doses in the microgram range. Although modern printers reduce the risk of reading errors, they may never be completely excluded. There is also the risk of false interpretation of this kind of symbols, when changing from one data carrier to another.
Dimensionless notations like percentages are only used in preparations for external application, and, by exception, in traditional combinations (normal saline 0.9 %, dextrose 5 %). If possible, a percentage should be indicated as m/m, m/v or v/v. According to the EMA the use of percentages should be discouraged, and the indication in mg per unit weight or per unit volume is to be preferred [8].
For substances that are standardised biologically, biological units are allowed. According to the international system (SI) only the International Unit may be abbreviated (IU). All other units should be written in full. In the UK the National Patient Safety Agency has recommended that even IU is not used but should be written out in full [11]. This was due to a number of deaths that have occurred owing to errors with abbreviations of (international) units. Also in other countries errors in dosing were discovered, due to lack of clarity in the necessary calculations from mg to USP units [12].
When labelling infusions or injections concentrations can be indicated in different ways, as in the following examples:
Potassium chloride 10 mmol = 10 ml (1 mmol/mL) or
Potassium chloride 10 mmol in 10 ml (1 mmol/mL) or
Potassium chloride 745 mg = 10 ml (74.5 mg/mL) or
Potassium chloride, K 1 mmol/mL, Cl 1 mmol/mL
Theophylline 200 mg = 1 ml
Heparin 5,000 IU = 1 ml
The EMA has developed recommendations for the expression of the strength of liquids [8], including parenteral medicines, where the way of expression also depends on the way of use, see Table 37.1.
Parenteral preparation | Preferred strength in name | Format |
|---|---|---|
Single dose (in case of total use of ampoule) | Total amount in container | z mg = z mL (1 mg/mL) |
Single dose (in case of partial use) | Amount per unit volume | X mg/mL (z mg = y mL) |
Multidose | Amount per unit volume | X mg/mL |
This means that for injections usually both the total amount of active substance and the concentration should be indicated, whereas for infusions the concentration and the total volume would be enough.
Note that in these recommendations only milligram is used, not mole. In practice for electrolytes the amount in moles is usually given as well. Doctors prescribe dosages of electrolytes based on blood concentrations that are given in mmoles.
37.3.2.9 Dose
Dose and frequency are indicated, if necessary at what times of the day. In case of variable doses (‘on demand’) the maximum use per 24 h and sometimes a maximum per week should be stated. Additional instructions may be needed, i.e. ‘Shake well before use’, or ‘Take with meals’, depending on the type of medicine. Of particular importance are warnings for staining or bleaching of clothes, or ‘highly flammable’. Lack of information of this kind may result in damage to the user or his property.
In some countries, e.g. Switzerland, a warning that the preparation contains ethanol is compulsory for oral mixtures with >0.7 %.
According to the European Regulation on Classification, Labelling and Packaging of Substances and Mixtures (CLP Regulation) [13] (see Sect. 26.6.3) hazard symbols are not compulsory in the labelling of medicines; for patients safety however, symbols referring to the risk of flammability and explosion are needed. For flammable substances the need depends mainly on their flashpoint.
As already mentioned, it is not always possible to fit all the information within the label. Examples are a dosage schedule for different times of the day, or a reduction scheme for corticosteroid treatment. In these situations additional oral and sometimes written explanation will be needed.
In the UK patients on steroid treatment receive a Steroid Treatment Card [14] so that they carry a warning against suddenly stopping therapy (and other information) in addition to the dosage schedule.
Dose administration aids, also called compliance aids, may help the patient to keep the overview. These weekly pill boxes are reusable boxes that allow medicines to be housed in grid like compartments, in preparation for sequential dosing according to a prescribed regime. Most boxes cater for up to 4 doses per day for 7 days. See also Sect. 37.7.4
37.3.2.10 Expiry Date and Beyond-Use Date
Expiry date and storage instructions are legally required on the label of all medicines. After the expiry date the manufacturer cannot guarantee the quality and safety of the product, no matter whether the package has been opened or not. For most patients however it is more important to know the expiration period after opening. This is the usage period, or period until the beyond-use date. Therefore the beyond-use date should be on the patient label, rather than the expiry date. In some countries pharmacists are required to affix beyond-use dates, supposing that the package will be opened shortly after dispensing. In licensed medicines, a beyond-use date is legally required only for special categories, such as parenteral medicines, ear drops and eye preparations.
For pharmacy preparations, usage periods per dosage form are given in Table 22.7. These general suggestions are mostly based on microbiological factors, and sometimes on physical properties. Often they may also be used for licensed medicines. See Sect. 22.7. for details.
The general advice for the maximum usage period only apply when there are no limits due to (chemical) instability of the active substance. For nationally standardised formulations, both shelf life and usage period usually have been determined. Examples are formulations in the German NRF (see Sect. 39.4.2), the Portuguese Galenic Formulary (PGF) [4] and the Dutch FNA [3], see Sect. 39.4.5.
The expiry date of pharmacy preparations depends on the date of preparation, the conditions under which the preparation is made, the type of container and the storage conditions. It should always be part of the label of stock preparations. In practice, it will not always be necessary for the patient to know this expiry date, as long as the beyond-use date lies before the expiry date, and the container is opened shortly after dispensing. Most important for the patient is that the label is clear and unambiguous on the maximum period of storage.
In treatments that should have a limited duration for pharmacotherapeutic reasons, the duration should be stated with the dosing scheme. Examples are strong topical steroids, or nose drops with decongestants.
37.3.2.11 Storage
Storage instructions that are important for the usage period should be on the label (e.g. Keep refrigerated, or Store at room temperature). Sometimes only the pharmacy stock needs to be kept in the refrigerator, while this is not necessary for the short period the patient uses the medicine. Examples are Acetic acid Ear drops and Atimos® or Foradil® aerosol. Both ear drops and preparations for inhalation should be at least at room temperature when used, because low temperatures can be unpleasant for the patient.
37.3.2.12 Where to Attach the Patient Label
In pharmacy preparations usually the primary container is labelled. The label on tubes should be near the cap, in order to keep it visible as long as possible during use of the product. A transparent film can be stuck on top of/over the label to protect it from the ointment in the tube. Cartridges that are filled for use in insulin pumps should not be labelled, as a label may interfere with the fitting of the cartridge into the pump. In such cases the label should be placed on the secondary packaging instead. Very small containers, such as eye ointment tubes or ampoules, can be labelled with a flag label with the minimal information that is required legally on the patient label. The remaining information should then appear on the label of a secondary package, or in a patient information leaflet (see Sect. 37.3.1).
For licensed medicines the patient label is often attached to the secondary packaging. This has the disadvantage that the label with the dosing information becomes lost if the patient discards the secondary package. But many primary packages, such as blisters or small vials, are unsuitable for labelling or it may simply not be allowed to open the package before dispensing. In such cases patient labels can only be attached to the secondary package; preferably a label on each single container of medicine when more than one is delivered.
37.4 Instructions on Use
37.4.1 Oral and Written Instructions
When dispensing medicines, oral instructions on use should be given in the pharmacy together with additional written information as appropriate. The way patients (or caregivers) receive instructions is one of the factors determining the quality of their manipulations with the medicine. Also it is important to try to understand a patient’s capabilities, language skills and situation. Research has shown that demonstrating, followed by copying by the patient, and additional written instructions all lead to better results, compared to just oral instructions [15]. This study focused on measuring liquid medicines with a measuring device, but the same applies to eye drops or inhalers.
Many countries have websites where patients can find instructions, or let them reproduce the instructions. Drug manufacturers give information on their websites and instruction videos for specific medicines. This product information is often not appropriate for drugs used in off-label situations, so in those cases the pharmacist’s advice is even more important. Information for specific patient groups can often be found on websites specialising in their disease (e.g. cancer or diabetes patients). But not all people have access to internet to access that information.
37.4.2 Packaging
Opening a package in the right way may require explanation (e.g. eye drop bottles, suppository strips, orally disintegrating tablets). Sometimes a user may prefer a specific container, for instance a jar instead of a tube for ointments.
37.4.3 Way of Use
37.4.3.1 Tablet Types
Solid oral dosage forms need explanation on the type. An effervescent tablet has to be dissolved before use, but small dispersible tablets could also be swallowed as a whole, with a glass of water. Taking the medicine with water is allowed, but not necessary in orally disintegrating tablets, which are designed to disintegrate on the tongue. Enteric coated tablets and dosage forms with controlled release usually must be swallowed whole. In Sects. 4.9 and 4.10 an overview is given of tablet types.
37.4.3.2 Dividing Tablets
Dividing or breaking tablets is another point of interest, and not only when it is mentioned in the prescription, or as a means of obtaining the prescribed dose. In many cases patients divide tablets on their own initiative, to ease swallowing or because they want to take a lower dose [16, 17]. Such actions are not always successful (Fig. 37.2).
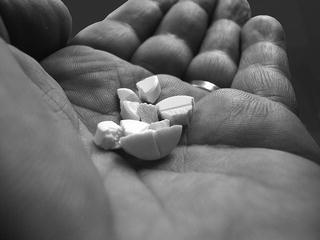

Fig. 37.2
A tablet in bits and pieces (Photo Marcel Terlouw, Argos. Source: Recepteerkunde 2009, ©KNMP)
The package leaflet does not always indicate whether a tablet may be divided, and the presence of a score line does not guarantee that splitting is possible or even allowed [18]. When in doubt, the pharmacist can refer to the product information for details of the formula. Enteric coatings, for instance, have characteristic components, see also Sect. 4.10.
When tablets may be subdivided and they have a break-mark, the halves have to comply with the requirements on the uniformity of mass of the Ph. Eur. under Subdivision of tablets [2]. Up till now these requirements only apply for scored tablets where subdivision is necessary to meet all the doses that are mentioned in the product information, not for break-marks intended to ease swallowing. There are also requirements proposed on the loss of mass by subdivision and the ease of breaking. In a Dutch study on a representative selection of tablets with a market authorisation only 24 % complied with the requirements on the uniformity of mass of the halves, and 34 % with the proposed standards on ease of subdivision [19]. These results were comparable to those of other studies on patient experiences with the performance of score lines [16, 17]. A so called tablet splitter can be useful (Fig. 37.3), although it does not always give better results than a kitchen knife or breaking by hand.


Fig. 37.3
Tablet splitters (Photo Luuk Dreijer. Source: Recepteerkunde 2009, ©KNMP)
That tablets break into unequal halves may not be clinically relevant, but patients tend to think it is important. Therefore it would be better if the requirements on uniformity of mass of the Ph. Eur. would apply to all tablets with a break-mark, whether this is needed for authorised doses or not.
In the meantime the pharmacist can try to reassure patients on this point, by using pharmacological knowledge.
37.4.3.3 Measuring Liquids
In pharmacy preparations packaging and measuring devices are part of the design of a product. In other words, attention should be paid to the feasibility of measuring the expected quantities with the supplied device from the container chosen.
For licensed medicines however, it is not uncommon that the dosing device in the package is unsuitable to measure the prescribed quantity to particular patients. In that case the pharmacist should supply a better measuring device. In young children administration of liquids with an oral syringe is often easier than with a measuring spoon. Cleaning instructions for oral syringes and pipettes are important when oily liquids are dispensed. A problem can be that the markings of the oral syringe may begin to wear off with normal use in a very short time. To measure the right dose of liquids for use in nebulisers, sometimes sterile syringes and needles are needed Fig. 37.4.
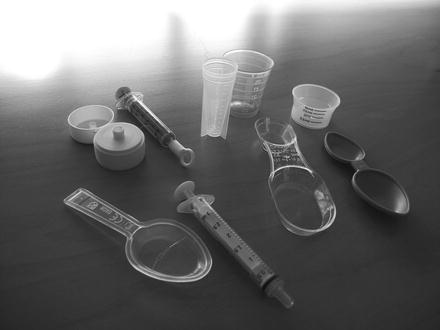

Fig. 37.4
Measuring spoons, cups and syringes (Photo Luuk Dreijer. Source: Recepteerkunde 2009, ©KNMP)
The devices supplied and the way the manipulations are carried out can greatly influence the dosing accuracy.
In a study from the USA, 14 % of the participants could measure 5 ml of an oral liquid within an acceptable range with a measuring cup, whereas 67 % could do this with a syringe. This percentage rose to nearly 100 % when the participants were given instruction and a demonstration with the syringe [20]. In 2005 EMA has published a guideline on the suitability of the graduation on measuring devices. Points of attention are the possibility to measure the minimal and the maximum dose, the dosing steps in relation to the advised dose, and the readability of the graduation [21]. In general it is recommended that a measuring cup or syringe should be filled to at least 40 % to obtain dosing with adequate accuracy (see Sect. 29.1.7). For very small volumes this will not always be possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


