65
Rational Prescribing & Prescription Writing
Once a patient with a clinical problem has been evaluated and a diagnosis has been reached, the practitioner can often select from a variety of therapeutic approaches. Medication, surgery, psychiatric treatment, radiation, physical therapy, health education, counseling, further consultation (second opinions), and no therapy are some of the options available. Of these options, drug therapy is by far the one most frequently chosen. In most cases, this requires the writing of a prescription. A written prescription is the prescriber’s order to prepare or dispense a specific treatment—usually medication—for a specific patient. When a patient comes for an office visit, the physician or other authorized health professional prescribes medications 67% of the time, and an average of one prescription is written per office visit because more than one prescription may be written at a single visit.
In this chapter, a plan for prescribing is presented. The physical form of the prescription, common prescribing errors, and legal requirements that govern various features of the prescribing process are then discussed. Finally, some of the social and economic factors involved in prescribing and drug use are described.
RATIONAL PRESCRIBING
Like any other process in health care, writing a prescription should be based on a series of rational steps.
1. Make a specific diagnosis: Prescriptions based merely on a desire to satisfy the patient’s psychological need for some type of therapy are often unsatisfactory and may result in adverse effects. A specific diagnosis, even if it is tentative, is required to move to the next step. For example, in a patient with a probable diagnosis of rheumatoid arthritis, the diagnosis and the reasoning underlying it should be clear and should be shared with the patient.
2. Consider the pathophysiologic implications of the diagnosis: If the disorder is well understood, the prescriber is in a much better position to offer effective therapy. For example, increasing knowledge about the mediators of inflammation makes possible more effective use of nonsteroidal anti-inflammatory drugs (NSAIDs) and other agents used in rheumatoid arthritis. The patient should be provided with the appropriate level and amount of information about the pathophysiology. Many pharmacies, websites, and disease-oriented public and private agencies (eg, Arthritis Foundation, American Heart Association, American Cancer Society, etc) provide information sheets suitable for patients.
3. Select a specific therapeutic objective: A therapeutic objective should be chosen for each of the pathophysiologic processes defined in the preceding step. In a patient with rheumatoid arthritis, relief of pain by reduction of the inflammatory process is one of the major therapeutic goals that identifies the drug groups that should be considered. Arresting the course of the disease process in rheumatoid arthritis is a different therapeutic goal, which might lead to consideration of other drug groups and prescriptions.
4. Select a drug of choice: One or more drug groups will be suggested by each of the therapeutic goals specified in the preceding step. Selection of a drug of choice from among these groups follows from a consideration of the specific characteristics of the patient and the clinical presentation. For certain drugs, characteristics such as age, other diseases, and other drugs being taken (because of the risk of duplicative therapy or drug-drug interactions) are extremely important in determining the most suitable drug for management of the present complaint. In the example of the patient with probable rheumatoid arthritis, it would be important to know whether the patient has a history of aspirin intolerance or ulcer disease, whether the cost of medication is an especially important factor and the nature of the patient’s insurance coverage, and whether there is a need for once-daily dosing. Based on this information, a drug would probably be selected from the NSAID group. If the patient does not have ulcer disease but does have a need for low-cost treatment, ibuprofen or naproxen would be a rational choice.
5. Determine the appropriate dosing regimen: The dosing regimen is determined primarily by the pharmacokinetics of the drug in that patient. If the patient is known to have disease of the organs required for elimination of the drug selected, adjustment of the average regimen is needed. For a drug such as ibuprofen, which is eliminated mainly by the kidneys, renal function should be assessed. If renal function is normal, the half-life of ibuprofen (about 2 hours) requires administration three or four times daily. The dose suggested in this book, drug handbooks, and the manufacturer’s literature is 400–800 mg four times daily.
6. Devise a plan for monitoring the drug’s action and determine an end point for therapy: The prescriber should be able to describe to the patient the kinds of drug effects that will be monitored and in what way, including laboratory tests (if necessary) and signs and symptoms that the patient should report. For conditions that call for a limited course of therapy (eg, most infections), the duration of therapy should be made clear so that the patient does not stop taking the drug prematurely and understands why the prescription probably need not be renewed. For the patient with rheumatoid arthritis, the need for prolonged—perhaps indefinite—therapy should be explained, including how to obtain refills. The prescriber should also specify any changes in the patient’s condition that would call for changes in therapy. For example, in the patient with rheumatoid arthritis, development of gastrointestinal bleeding would require an immediate change in drug therapy and a prompt workup of the bleeding. Major toxicities that require immediate attention should be explained clearly to the patient.
7. Plan a program of patient education: The prescriber and other members of the health team should be prepared to repeat, extend, and reinforce the information transmitted to the patient as often as necessary. The more toxic the drug prescribed, the greater the importance of this educational program. The importance of informing and involving the patient in each of the above steps must be recognized, as shown by experience with teratogenic drugs (see Chapter 59). Many pharmacies routinely provide this type of information with each prescription filled, but the prescriber must not assume that this will occur.
THE PRESCRIPTION
Although a prescription can be written on any piece of paper (as long as all of the legal elements are present), it usually takes a specific form. A typical printed prescription form for outpatients is shown in Figure 65–1.
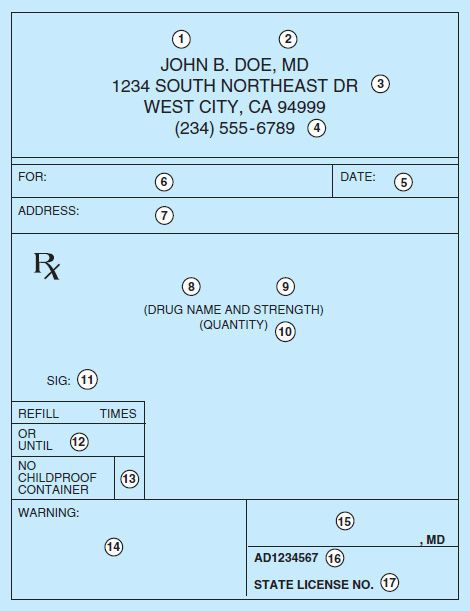
FIGURE 65–1 Common form of outpatient prescription. Circled numbers are explained in the text.
In the hospital setting, drugs are prescribed on a particular page of the patient’s hospital chart called the physician’s order sheet (POS) or chart order. The contents of that prescription are specified in the medical staff rules by the hospital’s Pharmacy and Therapeutics Committee. The patient’s name is typed or written on the form; therefore, the orders consist of the name and strength of the medication, the dose, the route and frequency of administration, the date, other pertinent information, and the signature of the prescriber. If the duration of therapy or the number of doses is not specified (which is often the case), the medication is continued until the prescriber discontinues the order or until it is terminated as a matter of policy routine, eg, a stop-order policy.
A typical chart order might be as follows:
3/12/14
10:30 a.m.
(1) Ampicillin 500 mg IV q6h 23times; 5 days
(2) Aspirin 0.6 g per rectum q6h prn temp over 101
[Signed] Janet B. Doe, MD
Thus, the elements of the hospital chart order are equivalent to the central elements (5, 8–11, 15) of the outpatient prescription.
ELEMENTS OF THE PRESCRIPTION
The first four elements (see circled numerals in Figure 65–1) of the outpatient prescription establish the identity of the prescriber: name, license classification (ie, professional degree), address, and office telephone number. Before dispensing a prescription, the pharmacist must establish the prescriber’s bona fides and should be able to contact the prescriber by telephone if any questions arise. Element [5] is the date on which the prescription was written. It should be near the top of the prescription form or at the beginning (left margin) of the chart order. Since the order has legal significance and usually has some temporal relationship to the date of the patient-prescriber interview, a pharmacist should refuse to fill a prescription without verification by telephone if too much time has elapsed since its writing.
Elements [6] and [7] identify the patient by name and address. The patient’s name and full address should be clearly spelled out.
The body of the prescription contains the elements [8] to [11] that specify the medication, the strength and quantity to be dispensed, the dosage, and complete directions for use. When writing the drug name (element [8]), either the brand name (proprietary name) or the generic name (nonproprietary name) may be used. Reasons for using one or the other are discussed below. The strength of the medication [9] should be written in metric units. However, the prescriber should be familiar with both systems now in use: metric and apothecary. For practical purposes, the following approximate conversions are useful:
1 grain (gr) = 0.065 grams (g), often rounded to 60 milligrams (mg)
15 gr = 1 g
1 ounce (oz) by volume = 30 milliliters (mL)
1 teaspoonful (tsp) = 5 mL
1 tablespoonful (tbsp) = 15 mL
1 quart (qt) = 1000 mL
1 minim = 1 drop (gtt)
20 drops = 1 mL
2.2 pounds (lb) = 1 kilogram (kg)
The strength of a solution is usually expressed as the quantity of solute in sufficient solvent to make 100 mL; for instance, 20% potassium chloride solution is 20 grams of KCl per deciliter (g/dL) of final solution. Both the concentration and the volume should be explicitly written out.
The quantity of medication prescribed should reflect the anticipated duration of therapy, the cost, the need for continued contact with the clinic or physician, the potential for abuse, and the potential for toxicity or overdose. Consideration should be given also to the standard sizes in which the product is available and whether this is the initial prescription of the drug or a repeat prescription or refill. If 10 days of therapy are required to effectively cure a streptococcal infection, an appropriate quantity for the full course should be prescribed. Birth control pills are often prescribed for 1 year or until the next examination is due; however, some patients may not be able to afford a year’s supply at one time; therefore, a 3-month supply might be ordered, with refill instructions to renew three times or for 1 year (element [12]). Some third-party (insurance) plans limit the amount of medicine that can be dispensed—often to only one month’s supply. Finally, when first prescribing medications that are to be used for the treatment of a chronic disease, the initial quantity should be small, with refills for larger quantities. The purpose of beginning treatment with a small quantity of drug is to reduce the cost if the patient cannot tolerate it. Once it is determined that intolerance is not a problem, a larger quantity purchased less frequently is sometimes less expensive.
The directions for use (element [11]) must be both drug-specific and patient-specific. The simpler the directions, the better; and the fewer the number of doses (and drugs) per day, the better. Patient noncompliance (also known as nonadherence, failure to adhere to the drug regimen) is a major cause of treatment failure. To help patients remember to take their medications, prescribers often give an instruction that medications be taken at or around mealtimes and at bedtime. However, it is important to inquire about the patient’s eating habits and other lifestyle patterns, because many patients do not eat three regularly spaced meals a day.
The instructions on how and when to take medications, the duration of therapy, and the purpose of the medication must be explained to each patient both by the prescriber and by the pharmacist. (Neither should assume that the other will do it.) Furthermore, the drug name, the purpose for which it is given, and the duration of therapy should be written on each label so that the drug may be identified easily in case of overdose. An instruction to “take as directed” may save the time it takes to write the orders out but often leads to noncompliance, patient confusion, and medication error. The directions for use must be clear and concise to prevent toxicity and to obtain the greatest benefits from therapy.
Although directions for use are no longer written in Latin, many Latin apothecary abbreviations (and some others included below) are still in use. Knowledge of these abbreviations is essential for the dispensing pharmacist and often useful for the prescriber. Some of the abbreviations still used are listed in Table 65–1.
TABLE 65–1 Abbreviations used in prescriptions and chart orders.
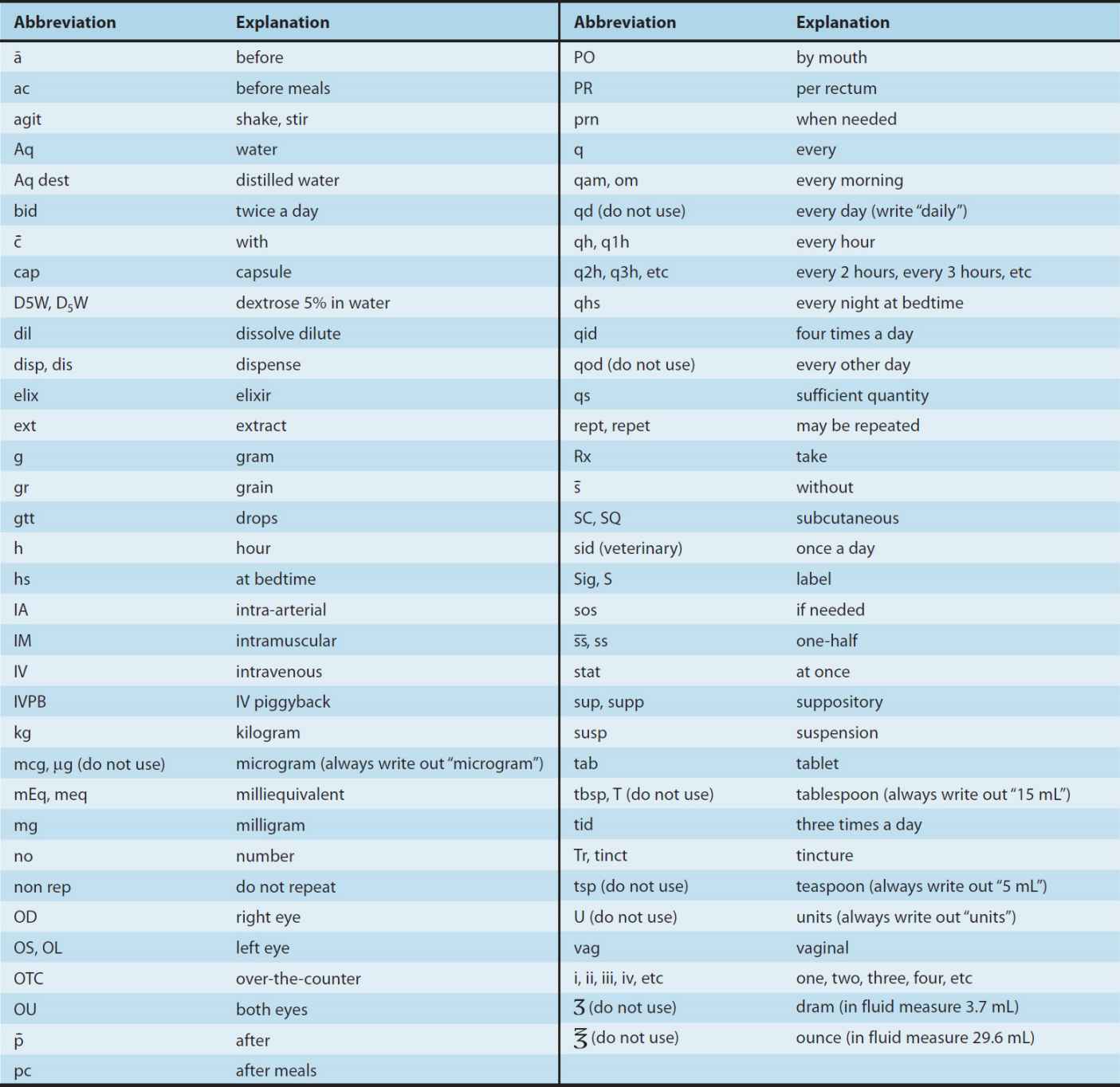
Note: It is always safer to write out the direction without abbreviating.
Elements [12] to [14] of the prescription include refill information, waiver of the requirement for childproof containers, and additional labeling instructions (eg, warnings such as “may cause drowsiness,” “do not drink alcohol”). Pharmacists put the name of the medication on the label unless directed otherwise by the prescriber, and some medications have the name of the drug stamped or imprinted on the tablet or capsule. Pharmacists must place the expiration date for the drug on the label. If the patient or prescriber does not request waiver of childproof containers, the pharmacist or dispenser must place the medication in such a container. Pharmacists may not refill a prescription medication without authorization from the prescriber. Prescribers may grant authorization to renew prescriptions at the time of writing the prescription or over the telephone or electronically. Elements [15] to [17] are the prescriber’s signature and other identification data such as National Provider Identification (NPI), Drug Enforcement Agency (DEA) number, or State License number.
PRESCRIBING ERRORS
Unfortunately, prescribing errors are common. Several groups provide online information regarding practices designed to reduce or document such errors, eg, Institute for Safe Medication Practices (ISMP; http://www.ismp.org/
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


