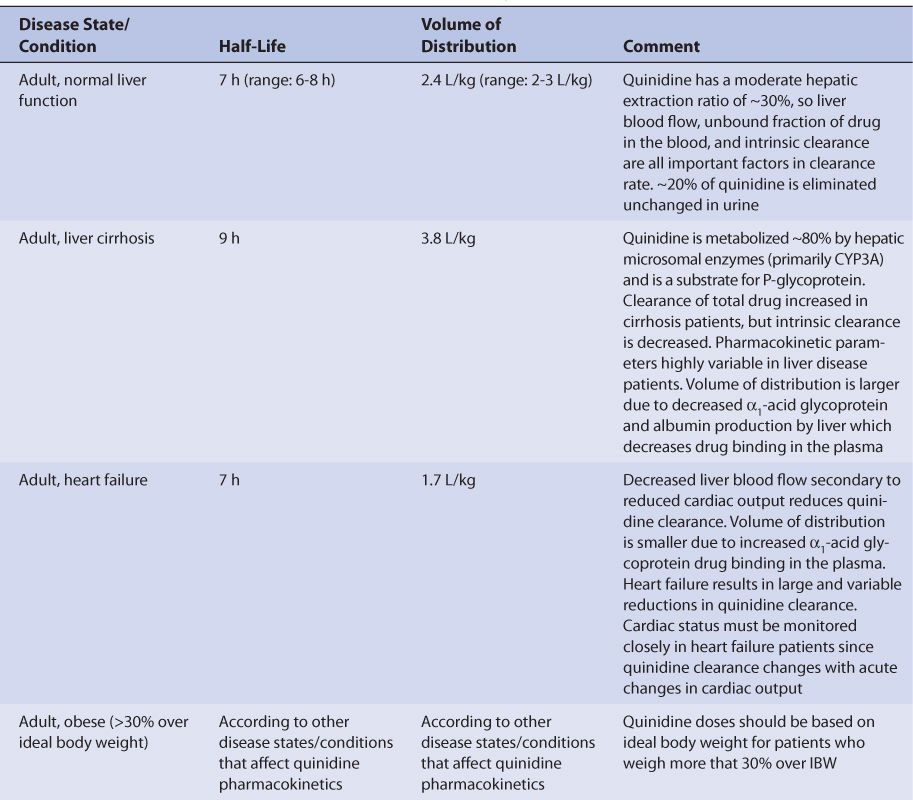
FIGURE 9-1 Quinidine serum concentrations after an intravenous dose (circles with solid line) and three different oral tablets (doses normalized to provide 200 mg of quinidine base systemically). After an intravenous dose, quinidine serum concentrations decline according to a two-compartment model, which demonstrates a distribution phase that lasts for 20-30 minutes postinjection. Immediate-release quinidine tablets (diamonds with dashed line) are rapidly absorbed and also show a distinct distribution phase. Extended-release quinidine gluconate (stars with dotted line) and quinidine sulfate (squares with solid line) have slower absorption profiles, so the drug has an opportunity to distribute to tissues while absorption is occurring. Because of this, no distribution phase is observed for these dosage forms.
The generally accepted therapeutic range for quinidine is 2-6 μg/mL. Quinidine serum concentrations above the therapeutic range can cause increased QT interval or QRS complex widening (>35%-50%) on the electrocardiogram, cinchonism, hypotension, high-degree atrioventricular block, and ventricular arrhythmias. Cinchonism is a collection of symptoms that includes tinnitus, blurred vision, lightheadedness, tremor, giddiness, and altered hearing which decreases in severity with lower quinidine concentrations. Gastrointestinal adverse effects such as anorexia, nausea, vomiting, and diarrhea are the most common side effects of quinidine therapy, can occur after both oral and intravenous quinidine routes of administration, but are not strongly correlated with specific serum levels. Quinidine therapy is also associated with syncope and torsade de pointes. Quinidine syncope occurs when ventricular tachycardia, ventricular fibrillation, or a prolongation of QT intervals occurs in a non–dose-dependent manner. Torsade de pointes (twisting of the points) is a form of polymorphic ventricular tachycardia preceded by QT interval prolongation. It is characterized by polymorphic QRS complexes that change in amplitude and length, giving the appearance of oscillations around the electrocardiographic baseline. Torsade de pointes can develop into multiple episodes of nonsustained polymorphic ventricular tachycardia, syncope, ventricular fibrillation, or sudden cardiac death. Hypersensitivity reactions to quinidine include rash, drug fever, thrombocytopenia, hemolytic anemia, asthma, respiratory depression, a systemic lupus-like syndrome, hepatitis, and anaphylactic shock.
Quinidine metabolites (3-hydroxyquinidine, 2′-quinidinone, quinidine-N-oxide, O-desmethylquinidine) have antiarrhythmic effects in animal models.15–18 Of these compounds, 3-hydroxyquinidine is the most potent (60%-80% compared to the parent drug) and achieves high enough serum concentrations in humans that its antiarrhythmic effects probably contribute to the clinical effects observed during quinidine treatment. Dihydroquinidine is an impurity contained in commercially available quinidine products that also has antiarrhythmic effects.19–21 Most products contain less than 10% of the labeled quinidine amount as dihydroquinidine. Clinicians should understand that all patients with “toxic” quinidine serum concentrations in the listed ranges will not exhibit signs or symptoms of quinidine toxicity. Rather, quinidine concentrations in the given ranges increase the likelihood that an adverse effect will occur.
For dose-adjustment purposes, quinidine serum concentrations are best measured as a predose or trough level at steady-state after the patient has received a consistent dosage regimen for 3-5 drug half-lives. Quinidine half-life varies from 6-8 hours in normal adults to 9-10 hours or more in adult patients with liver failure. If quinidine is given orally or intravenously on a stable schedule, steady-state serum concentrations will be achieved in about 2 days (5 • 8 h = 40 h).
CLINICAL MONITORING PARAMETERS
The electrocardiogram (ECG or EKG) should be monitored to determine the response to quinidine. The goal of therapy is suppression of arrhythmias and avoidance of adverse drug reactions. Electrophysiologic studies using programmed stimulation to replicate the ventricular arrhythmia or 24-hour ECG monitoring using a Holter monitor can be performed in patients while receiving a variety of antiarrhythmic agents to determine effective antiarrhythmic drug therapy.22
Because many quinidine therapeutic and side effects are not correlated with its serum concentration, it is often not necessary to obtain serum quinidine concentrations in patients receiving appropriate doses who currently have no arrhythmia or adverse drug effects. However, quinidine serum concentrations should be obtained in patients who have a recurrence of tachyarrhythmias, are experiencing possible quinidine side effects, or are receiving quinidine doses not consistent with disease states and conditions known to alter quinidine pharmacokinetics (see Effects of Disease States and Conditions on Quinidine Pharmacokinetics and Dosing section). Serum concentration monitoring can aid in the decision to increase or decrease the quinidine dose. For instance, if an arrhythmia reappears and the quinidine serum concentration is <6 μg/mL, increasing the quinidine dose is a therapeutic option. However, if the quinidine serum concentration is over 6 μg/mL, it is unlikely that a dosage increase will be effective in suppressing the arrhythmia and there is an increased likelihood that drug side effects may occur. Similarly, if a possible concentration-related quinidine adverse drug reaction is noted in a patient and the quinidine serum concentration is <2 μg/mL, it is possible that the observed problem may not be due to quinidine treatment and other sources can be investigated. While receiving quinidine, patients should be monitored for the following adverse drug effects: anorexia, nausea, vomiting, diarrhea, cinchonism, syncope, increased QT interval or QRS complex widening (>35%-50%) on the electrocardiogram, hypotension, high-degree atrioventricular block, ventricular arrhythmias, and hypersensitivity reactions (rash, drug fever, thrombocytopenia, hemolytic anemia, asthma, respiratory depression, a lupus-like syndrome, hepatitis, anaphylactic shock).
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
Quinidine is almost completely eliminated by hepatic metabolism (~80%).5,8 Hepatic metabolism is mainly via the CYP3A enzyme system. 3-Hydroxyquinidine is the primary active metabolite resulting from quinidine metabolism while dihydroquinidine is an active compound that is found as an impurity in most quinidine dosage forms. The hepatic extraction ratio of quinidine is about 30%, so quinidine is typically classified as an intermediate extraction ratio drug. Because of this, it is expected that liver blood flow, unbound fraction of drug in the blood, and intrinsic clearance will all be important factors influencing the clearance of quinidine. After oral administration, quinidine is subject to moderate first-pass metabolism by CYP3A contained in the liver and intestinal wall. Quinidine is also a substrate for P-glycoprotein. Approximately 20% of a quinidine dose is eliminated unchanged in the urine. Although there have been some reports that quinidine follows nonlinear pharmacokinetics, for the purposes of clinical drug dosing in patients, linear pharmacokinetic concepts and equations can be effectively used to compute doses and estimate serum concentrations.23
Two salt forms of quinidine are available. Quinidine sulfate contains 83% quinidine base, and quinidine gluconate contains 62% quinidine base. The gluconate salt is available for intravenous injection and oral use. Quinidine sulfate is available only for oral use. The oral bioavailability of both quinidine-based drugs is moderate and generally equals 70% reflecting first-pass metabolism in the intestinal wall and liver.4,8 Although quinidine injection can be given intramuscularly, this route of administration may lead to erratic absorption and serum concentrations.7
Plasma protein binding of quinidine in normal individuals is about 80%-90%.24–26 The drug binds to both albumin and α1-acid glycoprotein (AGP). AGP is classified as an acute-phase reactant protein that is present in lower amounts in all individuals but is secreted in large amounts in response to certain stresses and disease states such as trauma, heart failure, and myocardial infarction. In patients with these disease states, quinidine binding to AGP can be even larger resulting in an unbound fraction as low as 8%.
The recommended dose of quinidine is based on the concurrent disease states and conditions present in the patient that can influence quinidine pharmacokinetics. Quinidine pharmacokinetic parameters used to compute doses are given in the following section for specific patient profiles.
EFFECTS OF DISEASE STATES AND CONDITIONS ON QUINIDINE PHARMACOKINETICS AND DOSING
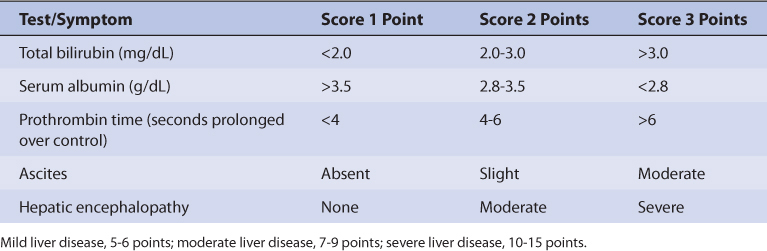
Normal adults without the disease states and conditions given later in this section and with normal liver function have an average quinidine half-life of 7 hours (range: 6-8 hours) and a volume of distribution for the entire body of 2.4 L/kg (V = 2-3 L/kg; Table 9-1).4–7,10,27–29 Disease states and conditions that change quinidine pharmacokinetics and dosage requirements may alter clearance and the volume of distribution. The elimination rate constant (k = 0.693/t1/2, where t1/2 is the half-life) and clearance (Cl = kV) can be computed from the aforementioned pharmacokinetic parameters.
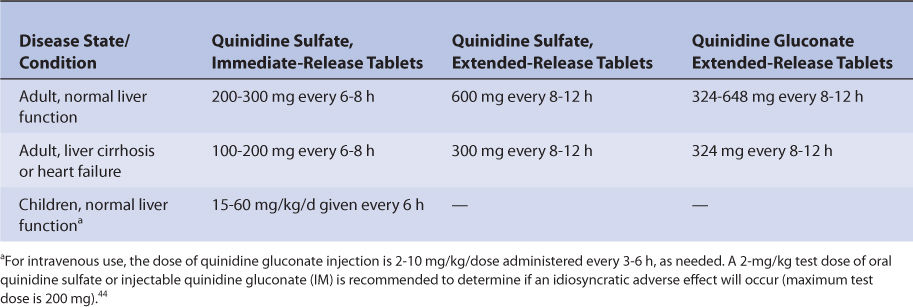
Patients with liver cirrhosis have increased quinidine clearance and volume of distribution which results in a prolonged average quinidine half-life of 9 hours.30,31 Clearance and volume of distribution are larger in patients with liver disease because albumin and α1-acid glycoprotein (AGP) concentrations are lower in these patients and result in reduced quinidine plasma protein binding (average V = 3.8 L/kg). The increased unbound fraction in the plasma allows more quinidine to enter the liver parenchyma where hepatic drug-metabolizing enzymes are present and leads to increased drug clearance. Decreased plasma protein binding also leads to higher unbound levels for a given total quinidine serum concentration. For example, a quinidine total serum concentration of 3 μg/mL would yield an unbound concentration of 0.3 μg/mL in a patient with normal plasma protein binding (3 μg/mL • 0.1 unbound fraction = 0.3 μg/mL), but an unbound concentration of 0.6 μg/mL in a cirrhosis patient with decreased plasma protein binding (3 μg/mL • 0.2 unbound fraction = 0.6 μg/mL). The significance of this difference in unbound concentrations has not been assessed in cirrhosis patients, but clinicians should bear it in mind when monitoring quinidine levels as only total serum concentrations are available from laboratories. The exact effect that liver disease has on quinidine pharmacokinetics is highly variable and difficult to accurately predict. It is possible for a patient with liver disease to have relatively normal or grossly abnormal quinidine clearance, volume of distribution, and half-life. An index of liver dysfunction can be gained by applying the Child-Pugh clinical classification system to the patient (Table 9-2).32 Child-Pugh scores are completely discussed in Chapter 3 (Drug Dosing in Special Populations: Renal and Hepatic Disease, Dialysis, Heart Failure, Obesity, and Drug Interactions), but will be briefly discussed here. The Child-Pugh score consists of five laboratory tests or clinical symptoms: serum albumin, total bilirubin, prothrombin time, ascites, and hepatic encephalopathy. Each of these areas is given a score of 1 (normal) to 3 (severely abnormal; see Table 9-2), and the scores for the five areas are summed. The Child-Pugh score for a patient with normal liver function is 5 while the score for a patient with grossly abnormal serum albumin, total bilirubin, and prothrombin time values in addition to severe ascites and hepatic encephalopathy is 15. A Child-Pugh score greater than 8 is grounds for a decrease of 25%-50% in the initial daily drug dose for quinidine. As in any patient with or without liver dysfunction, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Quinidine serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with liver cirrhosis.
Heart failure reduces quinidine clearance because of decreased hepatic blood flow secondary to compromised cardiac output (Table 9-3).8,9,33,34 Volume of distribution (V = 1.7 L/kg) is decreased because heart failure patients have elevated AGP serum concentrations which leads to increased quinidine plasma protein binding and decreased quinidine unbound fraction. Because both clearance and volume of distribution simultaneously decrease, patients with heart failure have an average quinidine half-life equal to 7 hours which is similar to a normal individual [t1/2 = (0.693 • ↓V)/↓Cl]. Increased plasma protein binding also leads to lower unbound levels for a given total quinidine serum concentration. For example, a quinidine total serum concentration of 3 μg/mL would yield an unbound concentration of 0.3 μg/mL in a patient with normal plasma protein binding (3 μg/mL • 0.1 unbound fraction = 0.3 μg/mL), but an unbound concentration of 0.15 μg/mL in a heart failure patient with increased plasma protein binding (3 μg/mL • 0.05 unbound fraction = 0.15 μg/mL). The clinical significance of this difference in unbound concentrations has not been assessed in heart failure patients. Obviously, the effect that heart failure has on quinidine pharmacokinetics is highly variable and difficult to accurately predict. It is possible for a patient with heart failure to have relatively normal or grossly abnormal quinidine clearance and half-life. For heart failure patients, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Quinidine serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with heart failure.
Patients with myocardial infarction may develop serious arrhythmias that require therapy with quinidine. After a myocardial infarction, serum AGP concentrations increase up to 50% over a 12-72-hour time period. As AGP serum concentrations increase, plasma protein binding of quinidine increases and the unbound fraction of quinidine decreases. Because quinidine is considered a moderate hepatic extraction ratio drug, a decline in the unbound fraction of quinidine in the plasma decreases quinidine clearance.
Patient age has an effect on quinidine clearance and half-life.16,35 For elderly patients over the age of 65, studies indicate that quinidine clearance is reduced, the volume of distribution is unchanged, and half-life is longer (average half-life = 10 hours) compared to younger subjects. A confounding factor found in quinidine pharmacokinetic studies conducted in older adults is the possible accidental inclusion of subjects that have subclinical or mild cases of the disease states associated with reduced quinidine clearance (heart failure, liver disease, etc). Additionally, most patients with serious arrhythmias studied in all of the previously mentioned investigations are older and those results include any influence of age. Thus, in most cases elderly patients are treated with quinidine according to the other disease states or conditions present that influence quinidine pharmacokinetics.
Because detailed studies have not been conducted in obese patients, ideal body weight should be used to compute initial doses of quinidine to avoid accidental overdose in overweight individuals (>30% above ideal body weight or IBW). Since only 20% of a quinidine dose is eliminated unchanged by the kidney, dosage adjustments for renal failure patients are usually not required.15,34 Quinidine is not appreciably removed by hemodialysis or peritoneal dialysis.36,37
DRUG INTERACTIONS
Quinidine has serious interactions with other drugs that are capable of inhibiting the CYP3A enzyme system.38 Because this isozyme is present in the intestinal wall and liver, quinidine serum concentrations may increase due to decreased clearance, decreased first-pass metabolism, or a combination of both. P-glycoprotein is also inhibited by quinidine, so drug transport may be decreased and cause drug interactions. Erythromycin, ketoconazole, and verapamil have been reported to increase quinidine serum concentrations or area under the concentration-time curve (AUC) by greater than 30%-50%. Other macrolide antibiotics (such as clarithromycin) or azole antifungals (such as fluconazole, miconazole, and itraconazole) that inhibit CYP3A probably cause similar drug interactions with quinidine. Cimetidine and amiodarone also have been reported to cause increases in quinidine concentrations or AUC of a similar magnitude. Drugs that induce CYP3A (phenytoin, phenobarbital, rifampin, rifabutin) decrease quinidine serum concentrations by increasing quinidine clearance and first-pass metabolism. It is important to remember that phenytoin has antiarrhythmic effects and is also classified as a type IB antiarrhythmic agent. Because of this, phenytoin and quinidine may have additive pharmacologic effects that could result in a pharmacodynamic drug interaction.
Although it is not a substrate for the enzyme, quinidine is a potent inhibitor of the CYP2D6 enzyme system.38–41 As little as 50 mg of quinidine can effectively turn an “extensive metabolizer” into a “poor metabolizer” for this isozyme. Because poor metabolizers of CYP2D6 substrates have little to none of this enzyme in their liver, the administration of quinidine does not result in a drug interaction in these individuals. Quinidine can markedly decrease the clearance β-adrenergic receptor blockers eliminated via CYP2D6 by 30% or more. Propranolol, metoprolol, and timolol have decreased clearance due to quinidine coadministration. Tricyclic antidepressants (nortriptyline, imipramine, and desipramine), haloperidol, and dextromethorphan also have increased serum concentrations when given with quinidine. Codeine is a prodrug with no analgesic effect that relies on conversion to morphine via the CYP2D6 enzyme system to decrease pain. When quinidine is given concomitantly with codeine, the conversion from codeine to morphine does not take place, and patients do not experience analgesia. A similar drug interaction may occur with dihydrocodeine and hydrocodone. Although it may not be reported in the literature for a specific compound, clinicians should consider that a drug interaction is possible between quinidine and any CYP2D6 substrate.
Quinidine increases digoxin serum concentrations 30%-50% by decreasing digoxin renal and nonrenal clearance as well as digoxin volume of distribution.38 The probable mechanisms of this drug interaction are inhibition of digoxin renal and hepatic P-glycoprotein (PGP) elimination and tissue-binding displacement of digoxin by quinidine. Antacids can increase urinary pH leading to increased renal tubular reabsorption of unionized quinidine and decreased quinidine renal clearance. Kaolin-pectin administration results in physical adsorption of quinidine in the gastrointestinal tract and decreased quinidine oral absorption. The pharmacologic effects of warfarin and neuromuscular blockers have been enhanced when given with quinidine.
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate quinidine therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. Literature-based Recommended Dosing is a very commonly used method to prescribe initial doses of quinidine. Doses are based on those that commonly produce steady-state concentrations in the lower end of the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient.
Pharmacokinetic Dosing Method
The goal of initial dosing of quinidine is to compute the best dose possible for the patient, given their set of disease states and conditions that influence quinidine pharmacokinetics and the arrhythmia being treated. In order to do this, pharmacokinetic parameters for the patient will be estimated using average parameters measured in other patients with similar disease state and condition profiles.
Half-Life and Elimination Rate Constant Estimate
Quinidine is predominately metabolized by liver. Unfortunately, there is no good way to estimate the elimination characteristics of liver-metabolized drugs using an endogenous marker of liver function in the same manner that serum creatinine and estimated creatinine clearance are used to estimate the elimination of agents that are renally eliminated. Because of this, a patient is categorized according to the disease states and conditions that are known to change quinidine half-life, and the half-life previously measured in these studies is used as an estimate of the current patient’s half-life (see Table 9-1). For a patient with moderate heart failure (NYHA CHF class III), quinidine half-life would be assumed to equal 7 hours, while a patient with severe liver disease (Child-Pugh score = 12) would be assigned an estimated half-life of 9 hours. To produce the most conservative quinidine doses in patients with multiple concurrent disease states or conditions that affect quinidine pharmacokinetics, the disease state or condition with the longest half-life should be used to compute doses. This approach will avoid accidental overdosage as much as currently possible. Once the correct half-life is identified for the patient, it can be converted into the quinidine elimination rate constant (k) using the following equation: k = 0.693/t1/2.
Volume of Distribution Estimate
As with the half-life estimate, the quinidine volume of distribution is chosen according to the disease states and conditions that are present (see Table 9-1). The volume of distribution is used to help compute quinidine clearance, and is assumed to equal 3.8 L/kg for liver disease patients, 1.7 L/kg for heart failure patients, and 2.4 L/kg for all other patients. For obese patients (>30% above ideal body weight), ideal body weight is used to compute quinidine volume of distribution. Thus, for a nonobese 80-kg patient without heart failure or liver disease, the estimated quinidine volume of distribution would be 192 L: V = 2.4 L/kg • 80 kg = 192 L. For a 150-kg obese patient with an ideal body weight of 60 kg and normal cardiac and liver function, the estimated quinidine volume of distribution is 144 L: V = 2.4 L/kg • 60 kg = 144 L.
Selection of Appropriate Pharmacokinetic Model and Equations
When given orally, quinidine follows a one- or two-compartment pharmacokinetic model (see Figure 9-1). When oral therapy is required, most clinicians utilize a sustained-release dosage form that has good bioavailability (F = 0.7), supplies a continuous release of quinidine into the gastrointestinal tract, and provides a smooth quinidine serum concentration-time curve that emulates an intravenous infusion when given every 8-12 hours. Because of this, a very simple pharmacokinetic equation that computes the average quinidine steady-state serum concentration (Css in μg/mL = mg/L) is widely used and allows maintenance dosage calculation: Css = [F • S (D/τ)]/Cl or D = (Css • Cl • τ)/(F • S), where F is the bioavailability fraction for the oral dosage form (F = 0.7 for most oral quinidine products), S is the fraction of the quinidine salt form that is active quinidine (S = 0.83 for sulfate: immediate-release tablets = 200, 300 mg, extended-release tablets = 300 mg; S = 0.62 for gluconate: extended-release tablets = 324 mg), D is the dose of quinidine salt in mg, and τ is the dosage interval in hours. Cl is quinidine clearance in L/h and is computed using estimates of quinidine elimination rate constant (k) and volume of distribution: Cl = kV. For example, for a patient with an estimated elimination rate constant equal to 0.099 h−1 and an estimated volume of distribution equal to 168 L, the estimated clearance would equal 16.6 L/h: Cl = 0.099h−1 • 168 L = 16.6 L/h.
Steady-State Concentration Selection
The general accepted therapeutic range for quinidine is 2-6 μg/mL. However, quinidine therapy must be individualized for each patient in order to achieve optimal responses and minimal side effects.
Literature-Based Recommended Dosing
Because of the large amount of variability in quinidine pharmacokinetics, even when concurrent disease states and conditions are identified, many clinicians believe that the use of standard quinidine doses for various situations are warranted. The original computation of these doses were based on the Pharmacokinetic Dosing method described in the previous section, and subsequently modified based on clinical experience. In general, the quinidine steady-state serum concentration expected from the lower end of the dosage range was 2-4 μg/mL and 4-6 μg/mL for the upper end of the dosage range. Suggested quinidine maintenance doses for adults and children are given in Table 9-4. A 25%-50% reduction in initial quinidine dose is suggested for patients with moderate-severe liver disease (Child-Pugh score ≥8) or moderate-severe heart failure (NYHA class II or greater). When more than one disease state or condition is present in a patient, choosing the lowest daily dose will result in the safest, most conservative dosage recommendation.
TABLE 9-4 Literature-Based Recommended Oral Quinidine Initial Dosage Ranges for Various Disease States and Conditions
To illustrate the similarities and differences between this method of dosage calculation and the Pharmacokinetic Dosing method, the same examples used in the previous section will be used.
USE OF QUINIDINE SERUM CONCENTRATIONS TO ALTER DOSES
Because of the large amount of pharmacokinetic variability among patients, it is likely that doses computed using patient population characteristics will not always produce quinidine serum concentrations that are expected or desirable. Because of pharmacokinetic variability, the narrow therapeutic index of quinidine, and the desire to avoid quinidine adverse side effects, measurement of quinidine serum concentrations can be a useful adjunct for patients to insure that therapeutic, nontoxic levels are present. In addition to quinidine serum concentrations, important patient parameters (electrocardiogram, clinical signs and symptoms of the arrhythmia, potential quinidine side effects, etc) should be followed to confirm that the patient is responding to treatment and not developing adverse drug reactions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree