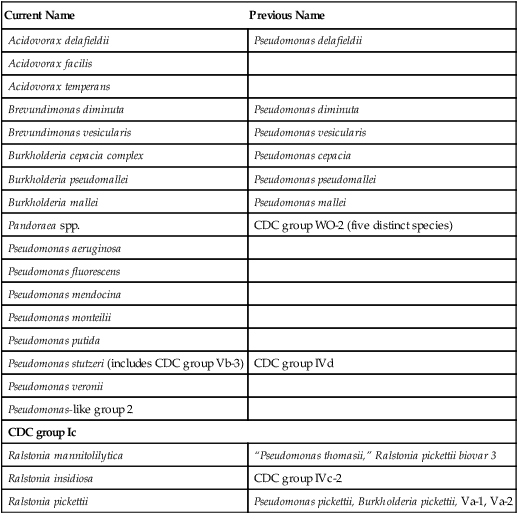
Chapter 22 1. Describe the normal sources (habitat) for Pseudomonas aeruginosa, Burkholderia cepacia, Burkholderia pseudomallei, and Burkholderia mallei, including the routes of transmission. 2. Identify the factors that contribute to the pathogenicity of P. aeruginosa and explain the physiologic mechanism for each. 3. List the various disease states associated with P. aeruginosa and Burkholderia spp. 4. Compare and contrast the Gram stain appearance of the gram-negative bacilli discussed in this chapter. 5. List the appropriate scheme for identifying P. aeruginosa. 6. Describe the media and chemical principle of each used, including differential and selective agars that aid the cultivation of Pseudomonas, Brevundimonas, and Ralstonia spp. 7. Describe the potential therapies for B. cepacia and B. pseudomallei and the concerns about optimal therapy. 8. Describe and identify the patterns of antibiotic resistance in P. aeruginosa. At one time, most of the species belonging to the genera Brevundimonas, Burkholderia, Ralstonia, and Acidovorax were members of the genus Pseudomonas. Organisms in these genera have many similar characteristics. They are aerobic, non–spore-forming, straight, slender, gram-negative bacilli with cells that range from 1 to 5 µm long and 0.5 to 1 µm wide. All species except B. mallei are motile, having one or several polar flagella. Members of these genera use a variety of carbohydrate, alcohol, and amino acid substrates as carbon and energy sources. Although they are able to survive and possibly grow at relatively low temperatures (i.e., as low as 4°C), the optimum temperature range for growth of most species is 30° to 37°C; that is, they are mesophilic. Burkholderia gladioli, Pseudomonas luteola, and Pseudomonas oryzihabitans are oxidase negative and are discussed in Chapter 21. Pseudomonas alcaligenes, Pseudomonas pseudoalcaligenes, Ralstonia paucula, Ralstonia gilardii, Comamonas spp. (including the former Pseudomonas testosteroni), and Delftia acidovorans (formerly Pseudomonas acidovorans) are not able to utilize glucose and are discussed in Chapter 25. Acidovorax facilis is MacConkey negative. Pseudomonas spp. are catalase positive. The organisms in this chapter are all oxidase-positive, grow on MacConkey agar, and oxidize glucose. Burkholderia spp. and Ralstonia pickettii are inhabitants of the environment and are not considered part of the normal human flora (Table 22-1). As such, their transmission usually involves human contact with heavily contaminated medical devices or substances encountered in the hospital setting. TABLE 22-1 The genera Pseudomonas and Brevundimonas comprise several environmental species that rarely inhabit human skin or mucosal surfaces. In the clinical setting, P. aeruginosa is the most commonly encountered gram-negative species that is not a member of the family Enterobacteriaceae and is an uncommon member of the normal human flora. The organism survives in various environments in nature and in homes and hospitals (see Table 22-1). Brevundimonas spp. are environmental and are encountered primarily in nature in water, soil, and on plants, including fruits and vegetables. Because of the ubiquitous nature of P. aeruginosa and Brevundimonas spp., the transmission of to humans can occur in a variety of ways. P. fluorescens, P. putida, and P. stutzeri are environmental inhabitants, but they are much less commonly found in clinical specimens than is P. aeruginosa. The other pseudomonads and Brevundimonas spp. listed in Table 22-1 are also environmental organisms. Because they are rarely encountered in patient specimens, the mode of transmission to humans remains uncertain. Because Burkholderia spp. and R. pickettii are uncommon causes of infection in humans, very little is known about what, if any, virulence factors they exhibit. Except for B. pseudomallei, the species listed in Table 22-2 generally are nonpathogenic for healthy human hosts. TABLE 22-2 Pathogenesis and Spectrum of Disease The remaining species listed in Table 22-2 are rarely encountered in human disease, and their clinical significance should be questioned when they are found in clinical specimens. P. aeruginosa also contains several genes involved in quorum sensing, a mechanism for detecting bacterial products in the immediate environment. When the growth of the organism or neighboring bacteria reaches a critical mass, the concentration of these “inducing” molecules reaches a level that activates transcription of virulence factors, including genes related to metabolic processes, enzyme production, and the formation of biofilm. Although many in vitro studies have examined biofilm formation, no clear evidence exists that demonstrates a clear role for biofilm in the organism’s pathogenesis. Although biofilm studies have been examined in the laboratory, it is evident that P. aeruginosa does not form the same type of biofilm in vivo as is seen on artificial surfaces. Biofilm production related to the overproduction of alginate and the mucoid phenotype isolated from patients with cystic fibrosis is associated with serious infections. P. aeruginosa forms microcolonies in tissue that are associated with quorum-sensing, biofilm-producing strains, which indicates that the quorum sensing is also linked to the formation of microcolonies. These microcolonies contain DNA, mucus, actin, and other products from dying bacterial and host cells. Additionally, P. aeruginosa can survive harsh environmental conditions and displays intrinsic resistance to a wide variety of antimicrobial agents, two factors that facilitate the organism’s ability to survive in the hospital setting (see Table 22-2). Even with the variety of potential virulence factors discussed, P. aeruginosa remains an opportunistic pathogen that requires compromised host defenses to establish infection. In normal, healthy hosts, infection is usually associated with events that disrupt or bypass protection provided by the epidermis (e.g., burns, puncture wounds, use of contaminated needles by intravenous drug abusers, eye trauma with contaminated contact lenses). The result is infections of the skin, bone, heart, or eye (see Table 22-2). No known virulence factors have been associated with P. fluorescens, P. putida, or P. stutzeri. When infections caused by these organisms occur, they usually involve a compromised patient exposed to contaminated medical materials. Such exposure has been known to result in infections of the respiratory and urinary tracts, wounds, and bacteremia (see Table 22-2). However, because of their low virulence, whenever these species are encountered in clinical specimens, their significance should be highly suspect. Similar caution should be applied whenever the other Pseudomonas spp. or Brevundimonas spp. listed in Table 22-2 are encountered. No special considerations are required for specimen collection and transport of organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport. Pseudomonas spp., Brevundimonas spp., Burkholderia spp., R. pickettii, and CDC group Ic grow well on routine laboratory media, such as 5% sheep blood agar and chocolate agar (Figure 22-1). Except for B. vesicularis, all usually grow on MacConkey agar. All four genera also grow well in broth-blood culture systems and common nutrient broths, such as thioglycollate and brain-heart infusion. Specific selective media, such as Pseudomonas cepacia (PC) agar or oxidative–fermentative base–polymyxin B–bacitracin–lactose (OFPBL) agar may be used to isolate B. cepacia from the respiratory secretions of patients with cystic fibrosis (Table 22-3). PC agar contains crystal violet, bile salts, polymyxin B, and ticarcillin to inhibit gram-positive and rapid-growing, gram-negative organisms. Inorganic and organic components, including pyruvate and phenol red, also are added. B. cepacia breaks down the pyruvate, creating an alkaline pH and resulting in a color change of the pH indicator (phenol red) from yellow to pink. OFPBL incorporates bacitracin as an added selective agent and uses lactose fermentation to differentiate isolates. B. cepacia ferments lactose and appears yellow, whereas nonfermenters appear green. Ashdown medium is used to isolate B. pseudomallei when an infection caused by this species is suspected. The medium contains crystal violet and gentamicin as selective agents to suppress the growth of contaminating organisms. Neutral red is incorporated into the medium and is taken up by the organism, making it distinguishable from other bacteria. TABLE 22-3
Pseudomonas, Burkholderia, and Similar Organisms
General Characteristics
Epidemiology
Burkholderia spp. and Ralstonia Pickettii
Species
Habitat (Reservoir)
Mode of Transmission
Burkholderia cepacia
Environmental (soil, water, plants); survives well in hospital environment; not part of normal human flora; may colonize respiratory tract of patients with cystic fibrosis
Exposure of medical devices and solutions contaminated from the environment; person-to-person transmission also documented
B. pseudomallei
Environmental (soil, streams, surface water, such as rice paddies); limited to tropical and subtropical areas, notably Southeast Asia; not part of human flora
Inhalation or direct inoculation from environment through disrupted epithelial or mucosal surfaces
B. mallei
Causative agent of glanders in horses, mules, and donkeys; not part of human flora
Transmission to humans is extremely rare; associated with close animal contact and introduced through mucous membranes or broken skin.
Ralstonia pickettii
Environmental (multiple sources); found in variety of clinical specimens; not part of human flora
Mode of transmission is not known; likely involves exposure to contaminated medical devices and solutions
Pseudomonas aeruginosa
Environmental (soil, water, plants); survives well in domestic environments (e.g., hot tubs, whirlpools, contact lens solutions) and hospital environments (e.g., sinks, showers, respiratory equipment); rarely part of normal flora of healthy humans
Ingestion of contaminated food or water; exposure to contaminated medical devices and solutions; introduction by penetrating wounds; person-to-person transmission is assumed to occur
P. alcaligenes, P. pseudoalcaligenes, Pseudomonas sp. CDC group 1, “P. denitrificans,” Pseudomonas-like group 2, and CDC group Ic
Environmental; not part of normal human flora
Uncertain. Rarely encountered in clinical specimens
P. fluorescens, P. putida, P. stutzeri, (including Vb-3), P. luteola, and P. mendocina
Environmental (soil and water); not part of normal human flora
Exposure to contaminated medical devices and solutions
Brevundimonas vesicularis and B. diminuta
Environmental; not part of normal human flora
Uncertain. Rarely encountered in clinical specimens
Acidovorax spp.
Environmental, soil; not part of human flora
Unknown. Rarely found in humans
Pseudomonas spp. and Brevundimonas spp.
Pathogenesis and Spectrum of Disease
Burkholderia spp. and Ralstonia Pickettii
Species
Virulence Factors
Spectrum of Disease and Infections
Burkholderia cepacia
Unknown. Binding of mucin from patients with cystic fibrosis may be involved. Intrinsic resistance to multiple antibiotics complicates therapy and may promote organism survival in hospital
Nonpathogenic to healthy human hosts; able to colonize and cause life-threatening infections in patients with cystic fibrosis or chronic granulomatous disease; other patients may suffer nonfatal infections of the urinary tract, respiratory tract, and other sterile body sites
B. pseudomallei
Unknown. Bacilli can survive within phagocytes
Wide spectrum from asymptomatic infection to melioidosis, of which there are several forms, including infections of the skin and respiratory tract, multisystem abscess formation, and bacteremia with septic shock
B. mallei
Unknown for human infections
Human disease is extremely rare. Infections range from localized acute or chronic suppurative infections of skin at site of inoculation to acute pulmonary infections and septicemia
Ralstonia pickettii
Unknown
Rarely encountered as cause of disease; nonpathogenic to healthy human host, but may be isolated from a variety of clinical specimens, including blood, sputum, and urine; when encountered environmental contamination should be suspected
Pseudomonas aeruginosa
Exotoxin A, endotoxins, proteolytic enzymes, alginate, and pili; intrinsic resistance to many antimicrobial agents
Opportunistic pathogen that can cause community- or hospital-acquired infections
Community-acquired infections: skin (folliculitis); external ear canal (otitis externa); eye, following trauma; bone (osteomyelitis), following trauma; heart (endocarditis) in IV drug abusers; and respiratory tract (patients with cystic fibrosis)
Hospital acquired infections: respiratory tract, urinary tract, wounds, bloodstream (bacteremia), and central nervous system
Key pathogen that infects lungs of cystic fibrosis patients
P. fluorescens, P. putida, and P. stutzeri (includes Vb-3)
Unknown. Infection usually requires patient with underlying disease to be exposed to contaminated medical devices or solutions
Uncommon cause of infection; have been associated with bacteremia, urinary tract infections, wound infections, and respiratory tract infections; when found in clinical specimen, significance should always be questioned
P. mendocina, P. alcaligenes, P. pseudoalcaligenes, Pseudomonas sp. CDC group 1, “P. denitrificans,” Pseudomonas-like group 2, and CDC group Ic
Unknown
Not typically known to cause human infections. P. mendocina has been isolated from a patient with endocarditis (R)
Brevundimonas vesicularis and B. diminuta
Unknown
Rarely associated with human infections. B. vesicularis is rare cause and of bacteremia in patients suffering underlying disease
Acidovorax spp.
Unknown
Rarely isolated from clinical specimens. Not implicated in human infections
Pseudomonas spp. and Brevundimonas spp.
Laboratory Diagnosis
Specimen Collection and Transport
Cultivation
Media of Choice
Organism
Medium
Appearance
Acidovorax delafieldii
BA
No distinctive appearance
Mac
NLF
Acidovorax facilis
BA
No distinctive appearance
Mac
Unable to grow
A. temperans
BA
No distinctive appearance
Mac
NLF
Brevundimonas diminuta
BA
Chalk white
Mac
NLF
B. vesicularis
BA
Orange pigment
Mac
NLF, but only 66% grow
Burkholderia cepacia complex
BA
Smooth and slightly raised; dirtlike odor
Mac
NLF; colonies become dark pink to red due to oxidation of lactose after 4-7 days
PC or OFPBL
Smooth
B. pseudomallei
BA
Smooth and mucoid to dry and wrinkled (may resemble P. stutzeri)
Mac
Ashdown
NLF
Dry, wrinkled, violet-purple
B. mallei
BA
No distinctive appearance
Mac
NLF
Pandoraea spp.
BA
No distinctive appearance
Mac
NLF
Pseudomonas aeruginosa
BA
Spreading and flat, serrated edges; confluent growth; often shows metallic sheen; bluish green, red, or brown pigmentation; colonies often beta–hemolytic; grapelike or corn tortilla–like odor; mucoid colonies commonly seen in patients with cystic fibrosis
Mac
NFL
P. fluorescens
BA
No distinctive appearance
Mac
NLF
P. mendocina
BA
Smooth, nonwrinkled, flat, brownish–yellow pigment
Mac
NLF
P. monteilii
BA
No distinctive appearance
Mac
NLF
P. mosselii
BA
No distinctive appearance
Mac
NLF
No acid production from xylose
P. putida
BA
No distinctive appearance
Mac
NLF
P. stutzeri and CDC group Vb–3
BA
Dry, wrinkled, adherent, buff to brown
Mac
NLF
P. veronii
BA
No distinctive appearance
Mac
NLF
Pseudomonas–like group 2
BAP
No distinctive appearance but colonies tend to stick to agar
Mac
NLF
CDC group Ic
BAP
No distinctive appearance
Mac
NLF
Ralstonia mannitolilytica
BAP
No distinctive appearance
Mac
NLF
R. pickettii
BAP
No distinctive appearance but may take 72 hr to produce visible colonies
Mac
NLF ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pseudomonas, Burkholderia, and Similar Organisms