Prostatic Intraepithelial Neoplasia
Rafael E. Jimenez, MD
Gladell P. Paner, MD
Mahul B. Amin, MD
Key Facts
Terminology
HGPIN: Noninvasive neoplastic transformation of lining epithelium of existing prostatic ducts and acini characterized by severe nuclear atypia
Etiology/Pathogenesis
TMPRSS–ERG fusion seen in 19% of HGPIN
Clinical Issues
HGPIN is present as isolated diagnosis in 4-16% of needle core biopsies and < 5% in transurethral resection specimens
Present in over 80% of prostate glands harboring adenocarcinoma vs. 43% of age-matched controls
Incidence increases with age, reaching up to 67% in 8th decade
Median risk of cancer following diagnosis of HGPIN is around 21% in more recent series
Microscopic Pathology
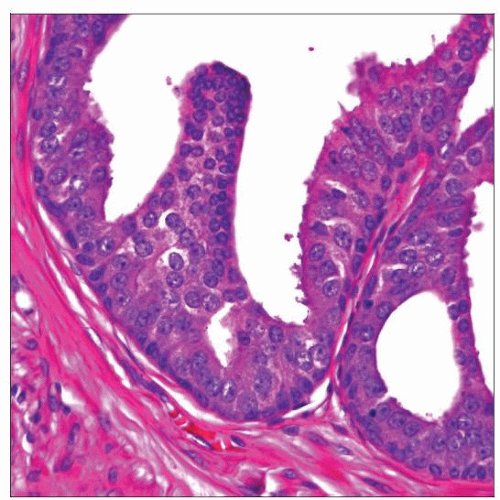
Preexistent ducts and acini are lined by crowded epithelial cells with abnormal cytologic features
Enlarged monomorphic nuclei, prominent nucleoli, hyperchromasia, nuclear overlap, amphophilic cytoplasm
Preserved or discontinuous basal cell layer may be readily identified on H&E or only with basal cell specific immunostains
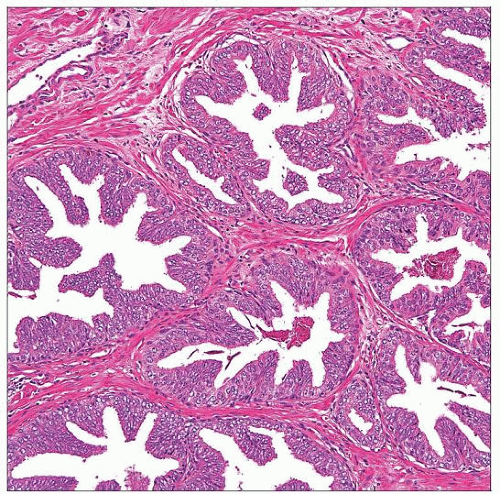
4 major architectural patterns: Tufted, micropapillary, cribriform, and flat
Other uncommon types: Signet ring, mucinous, foamy, inverted or “hobnail,” and small cell neuroendocrine
 HGPIN shows large and medium-sized glandular structures, which appear expanded and crowded but retain their rounded contours. Basal cell layer in HGPIN is often discernible on H&E stained sections. |
TERMINOLOGY
Abbreviations
Prostatic intraepithelial neoplasia (PIN)
PIN in routine surgical pathology terms usually refers to high-grade PIN (HGPIN)
Synonyms
Prostatic duct dysplasia
Definitions
PIN
Noninvasive neoplastic transformation of lining epithelium of preexisting prostatic ducts and acini, categorized into low-grade or high-grade PIN
HGPIN
PIN characterized by severe nuclear atypia (as in carcinoma) and varied, including more complex, architectural patterns
ETIOLOGY/PATHOGENESIS
Genetics
Provides evidence of association between HGPIN and prostate carcinoma (PCa)
TMPRSS–ERG fusion
Seen in 19% of HGPIN intermingling with cancer foci vs. 48.5% for clinically localized PCa
Aneuploid DNA seen in 32-58% of cases
Nuclear morphometric studies show characteristics intermediate between cancer and benign glands
Numeric alterations of chromosomes 7, 8, 10, 12, and Y common in both HGPIN and adenocarcinoma, though mean number higher in adenocarcinoma
Deletions of chromosome 8p most common allelic loss, detected in both HGPIN and adenocarcinoma
Increased expression of p16, p53, AMACR, Bcl-2, and MYC genes
Hypermethylation of glutathionine S-transferase
CLINICAL ISSUES
Epidemiology
Incidence
HGPIN is present as isolated diagnosis in up to 16% of needle core biopsies (usually 5-6%) and 1-5% in transurethral resection specimens
Present in 80-100% of prostate glands harboring adenocarcinoma vs. 43% of age-matched controls
Age
May be seen beginning in 3rd decade of life
Incidence increases with age, reaching up to 67% by 8th decade
Ethnicity
Lesion is usually more diffuse and presents earlier in African-Americans compared to Caucasians
Presentation
Asymptomatic, commonly encountered as incidental histologic finding
May have abnormal serum PSA level
Debatable if it is the cause of increased PSA; difficult to exclude undetected coexistent PCa
Treatment
Surgical approaches
No aggressive treatment (i.e., surgery, radiation) is warranted with diagnosis of HGPIN, unless concomitant adenocarcinoma is documented
Drugs
HGPIN has been studied as potential marker &/or target for chemoprevention of PCa
Risk of cancer
Previously, diagnosis of HGPIN without PCa would prompt rebiopsy, as 50-60% of cases would have PCa in subsequent biopsy
Contemporary data, in era of extended sampling biopsy, has shown that median risk of cancer following diagnosis of HGPIN is around 21%
This risk is not significantly different from risk following benign diagnosis (around 19%)
Thus, recommendations on follow-up of diagnosis of HGPIN are currently controversial
Patients with multifocal HGPIN (i.e., > 3 cores), bilateral HGPIN, and that associated with ASAP have higher risk of harboring concomitant PCa, and should be more aggressively followed
Other clinical or pathologic parameters do not appear to identify patients with higher risk of harboring PCa
Rebiopsy technique
PCa is identified with higher frequency in or adjacent to quadrant where HGPIN was detected
However, up to 45% of PCas are found in another sextant
Incidence of detection of subsequent PCa in patients with isolated HGPIN increases when rebiopsy is performed at 1 and 3 years
On rebiopsy, sampling should include all sextants
IMAGE FINDINGS
Ultrasonographic Findings
May be associated with hypoechoic lesion in peripheral zone
MACROSCOPIC FEATURES
General Features
Not associated with recognizable gross findings
Sections to Be Submitted
If only HGPIN (without invasive foci) detected in transurethral resection
Submit entire specimen for histologic evaluation or obtain deeper levels of block with HGPIN
Biopsy of peripheral zone may be an option, particularly in younger males
If only HGPIN (without invasive foci) detected in prostate needle biopsy specimen
May consider deeper levels if extensive or associated with atypical small acinar proliferation (ASAP)
MICROSCOPIC PATHOLOGY
Histologic Features
Preexisting ducts and acini, usually of medium to large size, lined by crowded epithelial cells with abnormal cytologic features
Hyperchromasia
Nuclear overlap
Enlarged relatively monomorphic nuclei
Prominent nucleoli (easily observed at 20x magnification)
Amphophilic cytoplasm
Diagnostic threshold varies as some individuals require all cells to be atypical and others require at least 10% of cells to have prominent nucleoli
Preserved or discontinuous basal cell layer may be readily identified on routine slides, or only with basal cell specific immunostains
4 major architectural patterns of HGPIN
Tufted (87%)
Stratification of acinar cells imparting luminal undulations or folds
Micropapillary (85%)
Nuclear stratification forming slender filiform projections and cellular budding
Cribriform (32%)
Complex intraluminal proliferation resulting in multiple irregular or round punched-out lumens
May show “cellular maturation,” wherein peripheral cells show greater nuclear atypia (i.e., nucleomegaly, prominent nucleoli) than cells at luminal aspect
Flat (25%)
Lacks significant cellular stratification, composed of only 1 or 2 cell layers
Other uncommon types
Multiple patterns may be seen concurrently
Variety of other architectural and cytologic features may be observed in HGPIN
Luminal cytoplasmic blebs, epithelial arches, cellular trabecular epithelial bars, “Roman bridges,” partial gland involvement, and basal cell layer disruption with glandular budding
Uncommonly, large cystic gland pattern, involvement in nodular hyperplasia and mucinous metaplasia
Variety of luminal features may be observed in HGPIN
Proteinaceous secretions, corpora amylacea, and exfoliated cells of PIN
Uncommonly, microcalcifications, and crystalloids; comedonecrosis is extremely rare
Predominant Pattern/Injury Type
Neoplastic
Predominant Cell/Compartment Type
Epithelial, glandular
Grade
Low-grade PIN
Tufted or micropapillary pattern
Nuclear crowding, stratification, and irregular spacing
Mild nuclear enlargement, with inconspicuous to rare prominent nucleoli
Diagnostic reproducibility is very low and has questionable relationship to PCa
Should not be diagnosed in needle core biopsies, as management and significance is uncertain
High-grade PIN
Cellular proliferation within medium to large glands
Increased basophilia or amphophilia readily detected at low power
Hyperchromasia, nuclear membrane irregularity, macronucleoli
Greater reproducibility among pathologists and more established relationship to concomitant or subsequent adenocarcinoma
ANCILLARY TESTS
Immunohistochemistry
Basal cell markers (p63, HMCK[34βE12]) highlight intact or frequently discontinuous basal cell layer around involved ducts
Due to discontinuous basal cell layer, these cells may not be apparent in particular plane of section, compounding differential diagnosis with PCa
AMACR variably stains acinar cells (56-100%)
PSA/PSAP(+) in acinar cells
Neuroendocrine HGPIN(+) for synaptophysin &/or chromogranin
DIFFERENTIAL DIAGNOSIS
Prostate Central Zone Glands
Show architectural complexity, including cribriforming and “Roman bridges,” but lack nuclear changes of HGPIN
Seminal Vesicle/Ejaculatory Duct Epithelium
No prominent nucleoli
More pleomorphism than HGPIN
Nuclear pseudoinclusions
Degenerative nuclear atypia
Intracellular coarse lipofuscin pigment
Prostate Glands with Reactive Atypia (Inflammation, Infarction, or Radiation)
Diagnosis of HGPIN should require more stringent criteria or should be questioned in areas of infarction, inflammation, or in previously radiated glands
Architectural features of HGPIN tend to be absent in mimics
Transitional Cell Metaplasia
Multilayered cells or solid nests that lack typical patterns of HGPIN
Uniform, smaller cells with nuclear grooves; secretory cell layer may be focally present
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree