Chapter 15 Prostaglandins and Other Eicosanoids
| Abbreviations | |
|---|---|
| COX | Cyclooxygenase |
| DP | D prostanoid |
| EP | E prostanoid |
| GI | Gastrointestinal |
| HPETE | Hydroxyperoxyeicosatetraenoic acid |
| LOX | Lipoxygenase |
| LTs | Leukotrienes |
| NSAID | Nonsteroidal antiinflammatory drug |
| PGs | Prostaglandins |
| TXs | Thromboxanes |
Therapeutic Overview
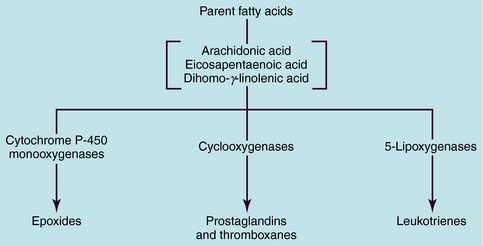
The term eicosanoid is used to represent a large family of endogenous compounds containing oxygenated unsaturated 20-carbon fatty acids and includes the prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs). The name PG was derived from the gland from which these compounds were first isolated, and the LTs derive their name from white blood cells and the inclusion of “trienes” or three conjugated double bonds. The PGs, TXs, and LTs are synthesized as shown schematically in Figure 15-1. Most pathways originate with the parent compound arachidonic acid, a major component of membrane phospholipids. Catalysis by cytochrome P450 monooxygenases produces epoxides, whereas the action of the cyclooxygenases (COXs) produces PGs and TXs, and that of the 5-lipoxygenases (5-LOX) produces LTs.
The PGs, TXs, and LTs exert profound effects on practically all cells and tissues, providing many potential targets for intervention in the treatment of disease. The PGs themselves are used as drugs to mimic effects they would produce if formed endogenously. In addition, many compounds such as the nonsteroidal antiinflammatory drugs (NSAIDs, see Chapter 36) and corticosteroids (see Chapter 39) produce their effects by inhibiting the formation of the PGs, whereas other compounds block the synthesis of the LTs. New drugs are also being introduced to block PG or LT receptors (see Chapter 16).
| Therapeutic Overview | ||
|---|---|---|
| Drug | Effect | Use |
| Corticosteroids | Block eicosanoid production | Inflammation |
| Prostaglandins | Increased blood flow and oxygenation by vessel relaxation | Neonatal defects |
| Penile erection | ||
| Increased uterine contraction | Induction of labor, abortifacient | |
| Reduced platelet aggregation | Peripheral vascular disease | |
| Reduce intraocular pressure | Glaucoma | |
| Suppress gastric acid secretion | Gastric ulcers | |
| Leukotriene antagonists | Block leukotriene receptor-mediated bronchoconstriction | Asthma |
| Leukotriene synthesis inhibitors | Inhibit lipoxygenase | Asthma |
| Nonsteroidal antiinflammatory drugs | Block prostaglandin synthesis | Pain, inflammation |
Mechanisms of Action
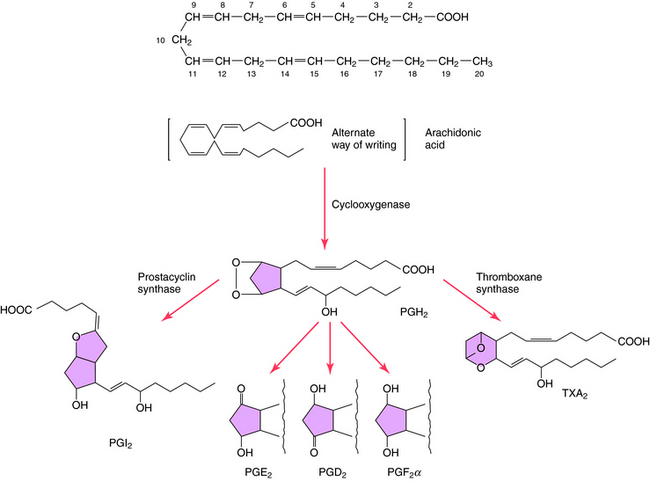
The structures and biosynthesis of PGs and TXs are shown in Figure 15-2. PGs are derived from essential fatty acids, usually arachidonic acid (C20:4). The numbering designation of arachidonic acid, 20:4, indicates 20 carbon atoms and 4 double bonds. The compounds that retain two double bonds in their alkyl side-chains are denoted by the subscript 2, and those that retain three double bonds are denoted by the subscript 3. Arachidonic acid and other fatty acids are cleaved from membrane phospholipids by the action of phospholipase A2 and are metabolized by three different types of enzymes: COXs, lipoxygenases, and cytochrome P450 monooxygenases.
COXs convert arachidonic acid into the PG endoperoxides PGG2 and PGH2. COXs are inhibited by NSAIDS such as aspirin and ibuprofen (see Chapter 36), leading to inhibition of PG and TX formation. Two distinct COXs have been described and have been designated as COX-1 and COX-2. COX-1 is constitutively expressed, whereas COX-2 is inducible and expressed in response to inflammatory mediators such as cytokines and lipopolysaccharides. Corticosteroids suppress the induction of COX-2 (see Chapter 39), suggesting that control of PG synthesis is involved in the antiinflammatory actions of these compounds. In addition, selective COX-2 inhibitors such as celecoxib (see Chapter 39) are useful in treating chronic inflammation, because they may cause less gastric disturbance than NSAIDS by allowing formation of cytoprotective PGE2 through COX-1.
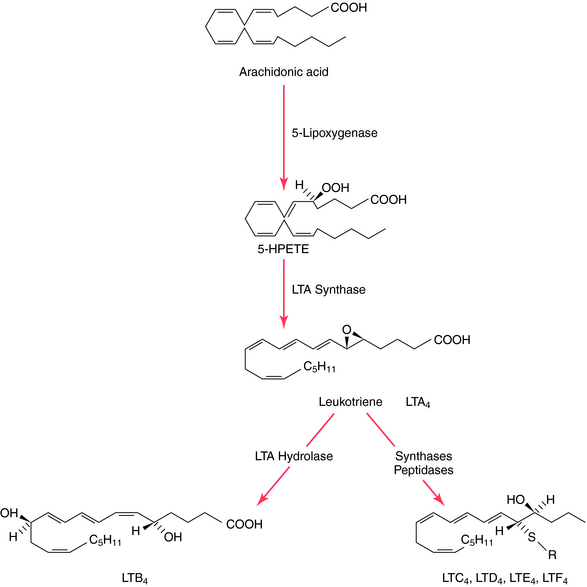
Like the PGs, the LTs are acidic lipids synthesized from essential fatty acids through the action of 5-LOX (Fig. 15-3), a pathway not inhibited by NSAIDs. Three major LOXs have been discovered, which catalyze incorporation of a molecule of O2 into the 5-, 12-, or 15-position of arachidonic acid, forming the corresponding 5-, 12-, or 15-hydroxyperoxyeicosatetraenoic (HPETE) acids. The 5-LOX pathway gives rise to the LTs. LTB4 has potent chemotactic properties for polymorphonuclear leukocytes, promoting adhesion and aggregation, whereas LTC4 and LTD4 are potent constrictors of peripheral lung airways and other vessels, including coronary arteries. The biologic activity of LTC4, LTD4, and LTE4 was previously termed “slow-reacting substance.”
PGs are synthesized and secreted in response to diverse stimuli; they are not stored. PGs and TXA2 act primarily as local hormones (autacoids), with their biological activities usually restricted to the cell, tissue, or structure where they are synthesized. Concentrations of PGE2 and PGF2α in arterial blood are very low because of pulmonary degradation, which normally removes more than 90% of these PGs from the venous blood as it passes through the lungs. Not all cells synthesize PGs; for example, there are segments of the nephron that lack COXs or show negligible capacity to transform added arachidonic acid to PGs. In contrast, COX is abundant within the vasculature, although the principal products vary longitudinally along the vasculature and cross-sectionally within the blood vessel wall (e.g., endothelium versus vascular smooth muscle). Within the coronary circulation, the larger blood vessels synthesize principally PGI2, whereas PGE2 predominates in microvessels.
PGs exert their effects by binding to specific cell surface receptors, which have been subdivided pharmacologically with respect to agonist potency and the signal transduction system to which they are coupled. All PG receptors are G-protein coupled receptors (see Chapter 1), which stimulate G-proteins to initiate transmembrane signaling. PGD2 activates D prostanoid (DP) receptors, PGE2 activates E prostanoid (EP) receptors, PGF2α activates F prostanoid (FP) receptors, and PGI2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree