Apolipoproteins
All lipoproteins have one or more apolipoprotein molecules embedded in their shell (see Fig. 42.1). Apolipoproteins, which constitute the protein component of lipoproteins, have three functions:
Classes of Lipoproteins
There are six major classes of plasma lipoproteins. Distinctions among classes are based on size, density, apolipoprotein content, transport function, and primary core lipids (cholesterol or TG). From a pharmacologic perspective, the features of greatest interest are lipid content, apolipoprotein content, and transport function.
Of the six major classes of lipoproteins, three are especially important in coronary atherosclerosis. These classes are named (1) very-low-density lipoproteins (VLDLs), (2) low-density lipoproteins (LDLs), and (3) high-density lipoproteins (HDLs). Properties of these classes are shown in Table 42.1.
TABLE 42.1
Properties of the Plasma Lipoproteins That Affect Atherosclerosis
| Lipoprotein Class | Major Core Lipids | Transport Function | Influence on Atherosclerosis |
| VLDL | Triglycerides | Delivery of triglycerides to nonhepatic tissues | Probably contribute to atherosclerosis |
| LDL | Cholesterol | Delivery of cholesterol to nonhepatic tissues | Definitely contribute to atherosclerosis |
| HDL | Cholesterol | Transport of cholesterol from nonhepatic tissues back to the liver | Protect against atherosclerosis |
Very-Low-Density Lipoproteins
VLDLs contain mainly triglycerides (and some cholesterol), and they account for nearly all of the TGs in blood. The main physiologic role of VLDLs is to deliver triglycerides from the liver to adipose tissue and muscle, which can use the TGs as fuel. Each VLDL particle contains one molecule of apolipoprotein B-100, which allows VLDLs to bind with cell-surface receptors and thereby transfer their lipid content to cells.
The role of VLDLs in atherosclerosis is unclear. Although several studies suggest a link between elevated levels of VLDLs and development of atherosclerosis, this link has not been firmly established. However, we do know that elevation of TG levels (above 500 mg/dL) increases the risk for pancreatitis.
Low-Density Lipoproteins
LDLs contain cholesterol as their primary core lipid, and they account for the majority (60%–70%) of all cholesterol in blood. The physiologic role of LDLs is delivery of cholesterol to nonhepatic tissues. LDLs can be viewed as byproducts of VLDL metabolism, in that the lipids and apolipoproteins that compose LDLs are remnants of VLDL degradation.
Cells that require cholesterol meet their needs through endocytosis (engulfment) of LDLs from the blood. The process begins with binding of LDL particles to LDL receptors on the cell surface. When cellular demand for cholesterol increases, cells synthesize more LDL receptors and thereby increase their capacity for LDL uptake. Accordingly, cells that are unable to make more LDL receptors cannot increase cholesterol absorption. Increasing the number of LDL receptors on cells is an important mechanism by which certain drugs increase LDL uptake and thereby reduce LDL levels in blood.
Of all lipoproteins, LDLs make the greatest contribution to coronary atherosclerosis. The probability of developing ASCVD is directly related to the level of LDLs in blood. Conversely, by reducing LDL levels, we decrease the risk for ASCVD. Accordingly, when cholesterol-lowering drugs are used, the main goal is to reduce elevated LDL levels. Multiple studies have shown that, by reducing LDL levels, we can arrest or perhaps even reverse atherosclerosis and can thereby reduce mortality from ASCVD. In fact, for each 1% reduction in the LDL level, there is about a 1% reduction in the risk for a major cardiovascular (CV) event.
High-Density Lipoproteins
Like LDLs, HDLs contain cholesterol as their primary core lipid, and they account for 20% to 30% of all cholesterol in the blood. In contrast to LDLs, whose function is delivery of cholesterol to peripheral tissues, HDLs carry cholesterol from peripheral tissues back to the liver. That is, HDLs promote cholesterol removal.
The influence of HDLs on ASCVD is dramatically different from that of LDLs. Whereas elevation of LDLs increases the risk for ASCVD, elevation of HDLs reduces the risk for ASCVD. That is, high HDL levels actively protect against ASCVD.
Low-Density Lipoprotein Versus High-Density Lipoprotein Cholesterol
The previous discussion shows that not all cholesterol in plasma has the same effect on ASCVD. As stated, a rise in cholesterol associated with LDLs increases the risk for ASCVD. In contrast, a rise in cholesterol associated with HDLs lowers the risk. Consequently, when speaking of plasma cholesterol levels, we need to distinguish between cholesterol that is associated with HDLs and cholesterol that is associated with LDLs. To make this distinction, we use the terms HDL cholesterol and LDL cholesterol. Because LDL cholesterol promotes atherosclerosis, it has been dubbed bad cholesterol. Conversely, because HDL seems to protect against atherosclerosis, it is often called good cholesterol or healthy cholesterol.
Role of Low-Density Lipoprotein Cholesterol in Atherosclerosis
LDLs initiate and fuel development of atherosclerosis. The process begins with transport of LDLs from the arterial lumen into endothelial cells that line the lumens of blood vessels. From there, they move into the space that underlies the arterial epithelium. When in the subendothelial space, components of LDLs undergo oxidation. This step is critical in that oxidized LDLs do the following:
• Attract monocytes from the circulation into the subendothelial space, after which the monocytes are converted to macrophages (which are critical to atherogenesis)
• Inhibit macrophage mobility, thereby keeping macrophages at the site of atherogenesis
• Undergo uptake by macrophages (macrophages do not take up LDLs that have not been oxidized)
• Are cytotoxic and hence can damage the vascular endothelium directly
As macrophages engulf more and more cholesterol, they become large and develop large vacuoles. When macrophages assume this form, they are referred to as foam cells. Foam cell accumulation beneath the arterial epithelium produces a fatty streak, which makes the surface of the arterial wall lumpy, causing blood flow to become turbulent. Continued accumulation of foam cells can eventually cause rupture of the endothelium, thereby exposing the underlying tissue to the blood. This results in platelet adhesion and formation of microthrombi. As the process continues, smooth muscle cells migrate to the site, synthesis of collagen increases, and there can be repeated rupturing and healing of the endothelium. The end result is a mature atherosclerotic lesion, characterized by a large lipid core and a tough fibrous cap. In less mature lesions, the fibrous cap is not strong, and hence the lesions are unstable and more likely to rupture. As a result, arterial pressure and shear forces (from turbulent blood flow) can cause the cap to rupture. Accumulation of platelets at the site of rupture can rapidly cause thrombosis and can thereby cause infarction. Infarction is less likely at sites of mature atherosclerotic lesions. The atherosclerotic process is depicted in Fig. 42.2.
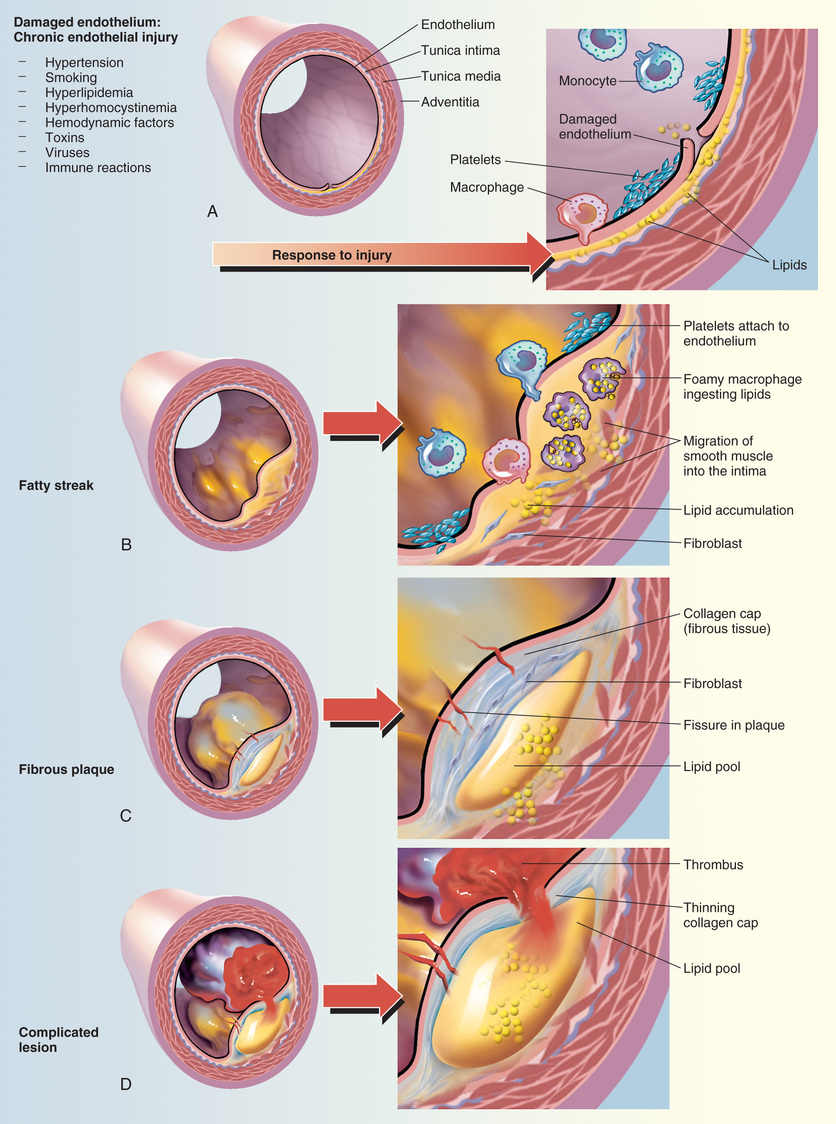
It is important to appreciate that atherogenesis involves more than just deposition of lipids. In fact, atherogenesis is now considered primarily a chronic inflammatory process. When LDLs penetrate the arterial wall, they cause mild injury. The injury, in turn, triggers an inflammatory response that includes infiltration of macrophages, T lymphocytes, and other potentially noxious chemicals (e.g., C-reactive protein [CRP]). In the late stage of the disease process, inflammation can weaken atherosclerotic plaque, leading to plaque rupture and subsequent thrombosis.
2013 American College of Cardiology/American Heart Association Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk
It is well established that high levels of cholesterol (primarily LDL cholesterol) cause substantial morbidity and mortality and that aggressive treatment can save lives. Accordingly, periodic cholesterol screening and risk assessment are recommended. If the assessment indicates ASCVD risk, lifestyle changes—especially diet and exercise—should be implemented. If ASCVD risk is high, LDL-lowering drugs should be added to the regimen.
In 1988, the National Cholesterol Education Program (NCEP) began issuing guidelines on cholesterol detection and management. The most recent update was issued in 2001 and amended in 2004, and new guidelines were developed in 2013 in partnership with the American College of Cardiology (ACC) and the American Heart Association (AHA). A summary of the 2013 guidelines—2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines—was published in the Journal of the American College of Cardiology and is available at http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437738.63853.7a.
Like earlier NCEP guidelines, the 2013 ACC/AHA cholesterol guideline focuses on the role of high cholesterol in ASCVD and stresses the importance of treatment. However, the new guideline focuses specifically on identifying patients who are most likely to benefit from cholesterol-lowering therapy instead of targeting specific cholesterol goals.
Cholesterol Screening
Adults
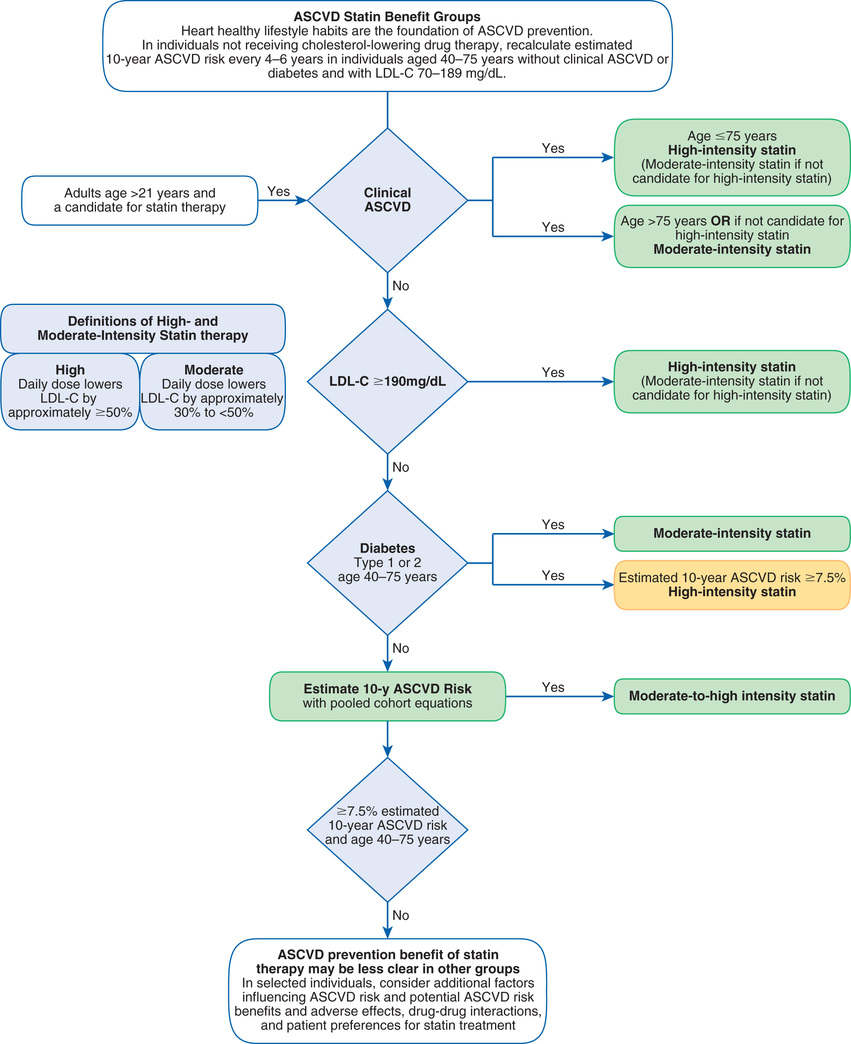
Management of high LDL cholesterol begins with screening, generally done every 5 years for adults older than 20 years. Because the 2013 update only addressed treatment, the ATP III guidelines (Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults) for screening and prevention remain current. ATP III recommends a thorough screening, consisting of total cholesterol, LDL cholesterol, HDL cholesterol, and TGs. Blood for these tests should be drawn after fasting. With the introduction of the new guidelines, patients should be considered for statin treatment if they fall into one of four different risk categories (Box 42.1).
Children and Adolescents
Elevated cholesterol in pediatric patients is a growing concern and is not addressed in the 2013 ACC/AHA blood cholesterol guidelines. However, it is addressed in other guidelines, including one created in 2011 by an expert panel appointed by the National Heart, Lung, and Blood Institute, and endorsed by the American Academy of Pediatrics. This report—Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents—is available at www.nhlbi.nih.gov/guidelines/cvd_ped/summary.htm#chap9.
The guideline recommends lipid screening for all children between ages 9 and 11 years, followed by another screen between ages 18 and 21 years. For children with a family history of high cholesterol or heart disease, screening should start sooner: between ages 2 and 8 years. Cholesterol classification for children and adolescents is presented in Table 42.2.
TABLE 42.2
NCEP Classification of Cholesterol Levels for Children and Adolescents*
| Category | Total Cholesterol (mg/dL) | LDL Cholesterol (mg/dL) |
| Acceptable | <170 | <110 |
| Borderline | 170–199 | 110–129 |
| Elevated | ≥200 | ≥130 |
If LDL cholesterol is high, all patients and their families should receive nutritional counseling. In addition, patients should focus on weight control and increased activity, as indicated. Should children use cholesterol-lowering drugs? For two reasons, the answer is, “Probably not.” First, these children are in no immediate danger: their risk for developing clinically significant ASCVD in the next 20 years is close to zero. And second, the only data from randomized, controlled trials were in children with familial hypercholesterolemia. No data exist showing that these drugs will improve outcomes when given to children with secondary lipid disorders.
PATIENT-CENTERED CARE ACROSS THE LIFE SPAN
Treating Dyslipidemia
| Life Stage | Patient Care Concerns |
| Infants | See later entry “Breastfeeding women.” |
| Children/adolescents | Lovastatin, simvastatin, pravastatin, and atorvastatin are approved for use in children. It is recommended to avoid statin use in children younger than 10 years. |
| Pregnant women | Statins are classified in FDA Pregnancy Risk Category X. They are contraindicated in pregnancy. Ezetimibe and fibrates are classified in Pregnancy Risk Category C; hence benefit should outweigh risk. |
| Breastfeeding women | Effects of statins, ezetimibe, and fibrates have not been studied in breastfeeding. Given the possibility of harm, benefit should outweigh risk. |
| Older adults | In patients 65 years and older, statins, compared with placebo, significantly reduced the risk for MI as well as the risk for stroke by 23.8%. However, the cost-benefit evaluation of treatment in older-adult people should be considered. |
Atherosclerotic Cardiovascular Disease Risk Assessment
Under the 2013 ACC/AHA guidelines, ASCVD risk assessment is directed at determining the patient’s absolute risk for developing clinical coronary disease over the next 10 years. The mode of intervention is then determined by the individual’s degree of risk.
Factors in Risk Assessment
To assess the ASCVD risk for an individual, we need three kinds of information. Specifically, we need to (1) identify ASCVD risk factors, (2) calculate 10-year ASCVD risk, and (3) identify ASCVD risk equivalents.
Identifying ASCVD Risk Factors
Major risk factors that modify LDL treatment goals include positive risk factors (advancing age, black race, hypertension, cigarette smoking, and low HDL cholesterol) and one negative risk factor (high HDL cholesterol). (LDL itself is not listed because the reason for counting these risk factors is to modify treatment of high LDL.)
We know that diabetes is a very strong predictor of developing ASCVD. Accordingly, we no longer consider diabetes to be a risk factor. Instead, for the purpose of risk assessment, diabetes is now considered an ASCVD risk equivalent. That is, having diabetes is considered equivalent to having ASCVD as a predictor of a major coronary event.
Calculating 10-Year ASCVD Risk
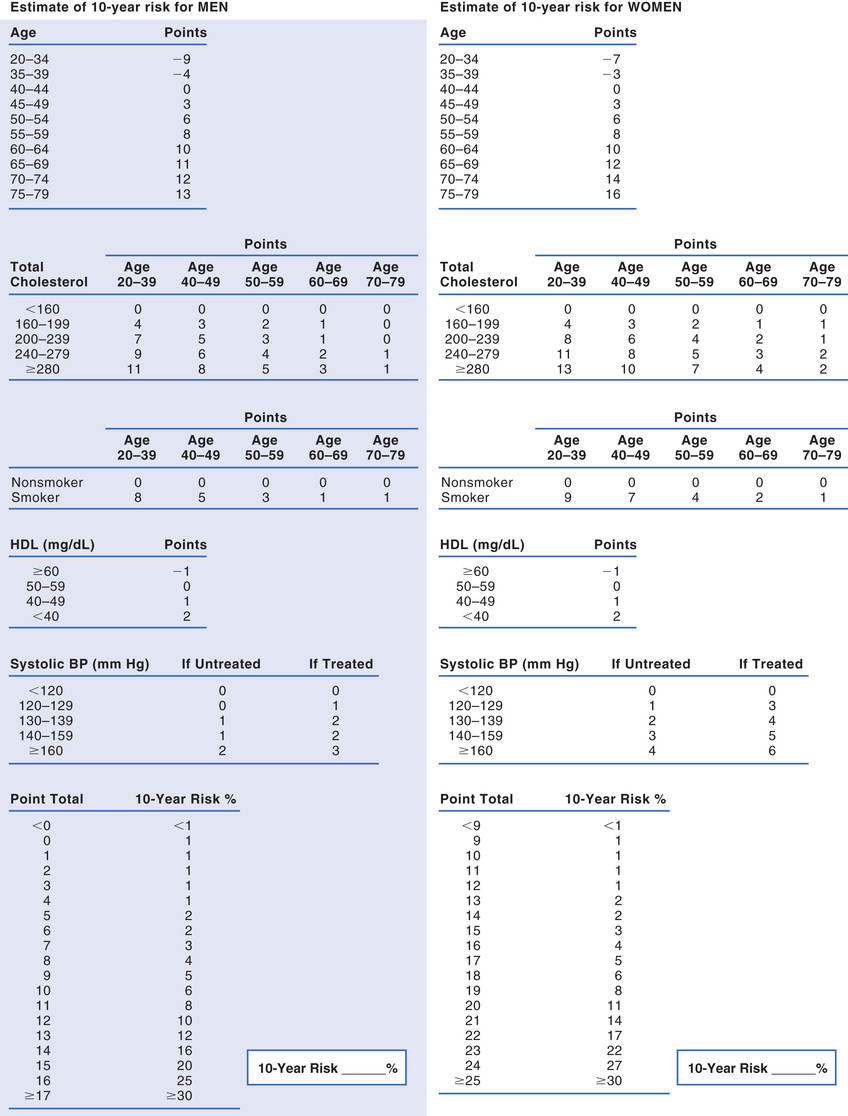
The 2013 ACC/AHA cholesterol guideline defines high ASCVD risk as 7.5% or greater. Some people are automatically in this risk group—specifically, those with existing ASCVD and those with diabetes. For all other people, 10-year risk must be calculated. The instrument employed most often is the Framingham Risk Prediction Score, which takes five factors into account: age, total cholesterol, HDL cholesterol, smoking status, and systolic blood pressure. These are similar to risk factors noted earlier. Framingham scores can be determined using either (1) the tables for men and women shown in Fig. 42.3 or (2) a web-based risk calculator, such as the one provided by the ACC/AHA at http://tools.cardiosource.org/ASCVD-Risk-Estimator/.
Identifying ASCVD Risk Equivalents
An ASCVD risk equivalent is a condition that poses the same risk for a major coronary event as does established ASCVD (i.e., more than 20% risk for a major event within 10 years). There are two basic ASCVD risk equivalents:
Identifying an Individual’s Atherosclerotic Cardiovascular Disease Risk Category
Under the 2013 ACC/AHA cholesterol guideline, there are four categories of patients who would benefit from statin treatment of cholesterol (see Box 42.1). Category assignment is based on (1) the presence or absence of ASCVD (or an ASCVD risk equivalent, such as diabetes), (2) the number of risk factors the individual has (other than high LDL cholesterol), and (3) the individual’s 10-year ASCVD score. Although this assessment sounds complicated, it’s not. Let’s consider the hypothetical case of Ralph J., and follow along by looking at Fig. 42.3. Mr. J. is 62 years old, hypertensive, and smokes—but, remarkably, his HDL cholesterol is high (above 60 mg/dL). He has no family history of premature ASCVD, does not have ASCVD himself, and does not have diabetes. His 10-year Framingham Risk Prediction Score is 11%. Because his estimated 10-year ASCVD score is greater than 7.5%, Mr. J. should be considered for moderate- to high-intensity drug therapy (Fig. 42.4). This is even easier if you use an online risk assessment tool, such as the ones available at http://www.framinghamheartstudy.org/risk-functions/index.php.
Final Note: Each Type of Dyslipidemia a Patient Has Contributes Independently to Atherosclerotic Cardiovascular Disease Risk
Patients are likely to have more than one type of dyslipidemia—for example, high LDL cholesterol combined with low HDL cholesterol and high TGs—and each of these disorders contributes independently to CV risk. This means that fixing just one of these problems will not eliminate the risk posed by the others. Accordingly, to get maximal risk reduction, we must correct all lipid abnormalities that are present.
Treatment of High Low-Density Lipoprotein Cholesterol
Treatment of high LDL cholesterol is based on the individual’s ASCVD risk category or the presence of other comorbidities such as diabetes. Treatment may be started with a high-intensity statin or a moderate-intensity statin depending on the patient’s risk factors (Table 42.3; see Fig. 42.4). To reduce LDL levels, the 2013 ACC/AHA guideline recommends two forms of intervention: (1) therapeutic lifestyle changes (TLCs) and (2) drug therapy. For some people, cholesterol can be reduced adequately with TLCs alone. Others require TLCs plus cholesterol-lowering drugs. Please note that drugs should be used only as an adjunct to TLCs—not as a substitute.
TABLE 42.3
High-, Moderate-, and Low-Intensity Statin Therapy
| High-Intensity Therapy | Moderate-Intensity Therapy | Low-Intensity Therapy |
| Daily dose lowers LCL-C on average by ≥50% | Daily dose lowers LDL-C on average by ~30% to <50% | Daily dose lowers LDL-C on average by <30% |
Atorvastatin: 40–80 mg Rosuvastatin: 20 mg | Atorvastatin: 10 mg Rosuvastatin: 10 mg Simvastatin: 20–40 mg Pravastatin: 40 mg Lovastatin: 40 mg | Simvastatin: 10 mg Pravastatin: 10–20 mg Lovastatin: 20 mg |
Therapeutic Lifestyle Changes
Therapeutic lifestyle changes are nondrug measures used to lower LDL cholesterol. TLCs focus on four main issues: diet, exercise, weight control, and smoking cessation. These measures are first-line treatment for LDL reduction and should be implemented before drug therapy. However, TLCs can be a challenge because some people do not eat healthier diets or exercise. Physical conditions such as arthritis can limit attempts at exercise, and economic and time limitations can be a barrier to healthier eating.
The TLC Diet
This diet has two objectives: (1) reducing LDL cholesterol and (2) establishing and maintaining a healthy weight. The central feature of the diet is reduced intake of cholesterol and saturated fats: individuals should limit intake of cholesterol to 200 mg/day or less and intake of saturated fat to 7% or less of total calories. Intake of trans fats—found primarily in snack crackers, commercial baked goods, and fried foods—should be minimized. (Many food manufacturers are adding “no trans fat” labels to their product labels, making shopping somewhat easier.)
If the basic TLC diet fails to lower LDL cholesterol adequately, ATP III recommends two additional measures: increased intake of soluble fiber (10–25 g/day; oatmeal is a good source) and increased intake of plant stanols and sterols (2 g/day). Plant stanols and sterols are cholesterol-lowering chemicals found (albeit in very small amounts) in certain vegetable oils (e.g., canola), nuts (walnuts are a good source), certain fruits, and most beans and many other vegetables. They are also found in some of the cholesterol-lowering margarines, commonly advertised as “buttery spreads” (see later section “Plant Stanol and Sterol Esters”).
Exercise
An inactive lifestyle carries an increased risk for ASCVD. Conversely, participating in regular exercise lowers ASCVD risk. Running and swimming, for example, can decrease LDL cholesterol and elevate HDL cholesterol, thereby reducing risk. In addition, exercise can reduce blood pressure, improve overall CV performance, and decrease insulin resistance (important because many people with high cholesterol also have diabetes). Accordingly, ATP III encourages regular physical activity (defined as 30–60 minutes of activity on most days). Improvements in the plasma lipid profile depend more on the total time spent exercising than on the intensity of exercise or improvements in fitness.
Smoking Cessation
Smoking cigarettes raises LDL cholesterol and lowers HDL cholesterol, thereby increasing the risk for ASCVD. Smokers should be strongly encouraged to quit—and nonsmokers should be urged not to start. Drugs to aid smoking cessation are discussed in Chapter 32.
Weight Control
Weight loss can reduce both LDL cholesterol and ASCVD risk. This is especially important for people with metabolic syndrome (see later).
Drug Therapy
Drugs are not the first-line therapy for lowering LDL cholesterol. Rather, drugs should be employed only if TLCs fail to reduce LDL cholesterol to an acceptable level—and then only if the combination of elevated LDL cholesterol and the patient’s ASCVD risk category justify drug use. When drugs are used, it is essential that lifestyle modification continues because the beneficial effects of diet and drugs are additive; drugs alone may be unable to achieve the LDL goal. It is important to note that the principal benefit of drug therapy is primary prevention: Drugs are much better at preventing or slowing ASCVD than at promoting regression of established coronary atherosclerosis. Furthermore, because LDL cholesterol levels will return to pretreatment values if drugs are withdrawn, treatment must continue lifelong. Patients should be made aware of this requirement.
Table 42.4 shows properties of the drug families used to lower LDL cholesterol. The most effective agents are the HMG-CoA reductase inhibitors (e.g., atorvastatin [Lipitor]), usually referred to simply as statins. A newer class of immunologics called monoclonal antibodies can also be used to treat high cholesterol. Lesser used alternatives are bile acid sequestrants (e.g., cholestyramine) and niacin (nicotinic acid). Although fibrates are listed in Table 42.4, these drugs are used primarily to reduce levels of TGs—not LDLs. Treatment is initiated with a single drug, almost always a statin. If the statin is ineffective, a bile acid sequestrant or niacin can be added to the regimen.
TABLE 42.4
Drugs Used to Improve Plasma Levels of LDLs, HDLs, and Triglycerides
| Drug Class | Effect on LDLs, HDLs, and TGs | Common or Serious Adverse Effects | Contraindications | Clinical Trial Results |
| HMG-CoA reductase inhibitors (statins) | Absolute: Relative: • Concurrent use of certain drugs* | Reduced major coronary events, stroke, ASCVD deaths, need for coronary procedures, and total mortality | ||
| Bile acid sequestrants | Absolute: Relative: | Reduced major coronary events and ASCVD deaths | ||
| Niacin (nicotinic acid) | Absolute Relative: | Reduced major coronary events and, possibly, reduced mortality | ||
| Fibrates | Absolute: | Reduced major coronary events | ||
| Ezetimibe | Absolute: | Effect on coronary events and mortality has not been established | ||
| PCSK9 inhibitors | History of hypersensitivity to Repatha or Praluent | Effect on coronary events and mortality has not been established |
In addition to lowering LDL cholesterol, drugs may be used to raise HDL cholesterol. The most effective agents are niacin and the fibrates. However, virtually all of the drugs that we use to lower LDL cholesterol have the added benefit of increasing HDL cholesterol, at least to some degree. This rise of HDL, therefore, can be considered a beneficial “side effect.”
Secondary Treatment Targets
Metabolic Syndrome
The term metabolic syndrome (also known as syndrome X) refers to a group of metabolic abnormalities associated with an increased risk for ASCVD and type 2 diabetes. The metabolic abnormalities involved are high blood glucose, high TGs, high apolipoprotein B, low HDL, small LDL particles, a prothrombotic state, and a proinflammatory state. Hypertension is both common and important.
According to a joint scientific statement—issued by the International Diabetes Federation Task Force on Epidemiology and Prevention; the National Heart, Lung, and Blood Institute; the American Heart Association; the World Heart Federation; the International Atherosclerosis Society; and the International Association for the Study of Obesity—the metabolic syndrome is diagnosed when three or more of the following are present:
• High TG levels—150 mg/dL or higher (or undergoing drug therapy for high TGs)
• Low HDL cholesterol—below 40 mg/dL for men or below 50 mg/dL for women (or undergoing drug therapy for reduced HDL)
• Hyperglycemia—fasting blood glucose 100 mg/dL or higher (or undergoing drug therapy for hyperglycemia/diabetes mellitus)
• High blood pressure—systolic 130 mm Hg or higher and/or diastolic 85 mm Hg or higher (or undergoing drug therapy for hypertension)
• Waist circumference 40 inches or more for most men or 35 inches or more for most women (these limits can vary depending on ethnicity, country, or geographic region within a country)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree