Fig. 17.1
Processes that determine bioavailability at extravascular administration routes
Release of the active substance from the dosage form facilitated by disintegration or dissolution or both (= pharmaceutical availability)
Pre systemic elimination, membrane passage, first-pass metabolism, and appearance of the active substance in the systemic circulation (= biological availability)
An active substance generally exerts its effect through defined molecular interactions with a receptor. As a prerequisite, the substance needs to be dissolved and reach the receptor. For a local effect, the substance is usually administered in close proximity to the desired target and should penetrate into body tissues as little as possible. However, when a systemic effect is desired, the substance has to reach the blood circulation system for distribution. For extravascular administration routes, this requires membrane passage and may expose the substance to various pre systemic elimination mechanisms, including metabolism and efflux transporters such as P-glycoprotein.
In the request for a pharmacy preparation, the physician often prescribes the administration route. Pharmacists have to critically evaluate whether the proposed route is appropriate to reach the desired bioavailability. For example, conventional oral medications may not be suitable for patients with a nasogastric feeding tube, children, or nauseous patients and alternative routes such as parenteral, rectal, or nasal have to be considered (see also Sect. 37.6.3). When adapting a dosage form or administration route to special needs of a patient, the pharmacist is required to consider safety aspects. It is important to recognise that an active substance approved for oral administration may never have been tested for safety and efficacy when administered via a different route such as the cutaneous application. Formally, at least, local toxicity studies are then required. Furthermore, excipients that are approved for tablet formulations may not be used in parenteral formulations. These examples illustrate that when a different administration route is chosen there might be a need to reassess the appropriate selection of excipients. In some cases, additional safety studies will be required.
“The European – United States Paediatric Formulation Initiative (Eu-US PFI) has established that there is a pressing need for a single authoritative comprehensive database of adverse effects of excipients for paediatrics. Safety and Toxicity of Excipients for Paediatrics (STEP) Database holds all the animal toxicity and human health data, regulatory information and toxicological reviews of excipients. STEP acts as repository for all the scientific communities to share the data for better understanding and paediatric medicines development” (European Paediatric Formulation Initiative. STEP Database. See [3]).
The selection of an adequate dosage form should also consider limitations associated with the preparation method. For instance, if an active substance is harmful to the operator, the pharmacist may decide to prepare a liquid dosage form rather than a solid dosage form to avoid exposure to the harmful agent via inhalation (see Sect. 26.7.1).
Physico-chemical properties of the active substance influence to a large extent the administration routes and dosage forms that are feasible. Relevant properties include solubility, partition coefficient (log P), pKa, membrane passage, metabolic stability, and half-life. Conversely, the administration route and formulation influence safety and efficacy of the active substance. This cause-effect relationship implies that formulations designed for different or even the same route of administration may not be interchangeable. Chapters 4–14 discuss these (im)possibilities in more detail, for different administration routes.
Although principally only valid for oral administration, it may be useful to assess the biopharmaceutical feasibility of any desired route of administration by the Biopharmaceutics Classification System (BCS, see e.g. [4]) (Fig. 17.2).
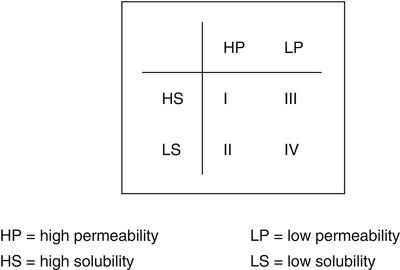

Fig. 17.2
Biopharmaceutics classification system
The BCS classifies active substances into four classes:
Class I: high solubility, high permeability
Class II: low solubility, high permeability
Class III: high solubility, low permeability
Class IV: low solubility, low permeability
17.4.1 Solubility
The relationship between the fluid volume available at the site of administration and the dose administered will determine whether or not solubility and dissolution rate of the active substance are adequate. Mathematically, this critical interaction is described by the dimensionless dose number (see also Sect. 16.1.5):
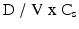
where:
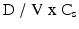
(17.1)
D = dose
V = (aqueous) volume available for dissolution
Cs = solubility in water
If the dose number < 0.1, solubility of the active substance is not considered limiting. If the dose number > 10, it is predicted that limited solubility of the active substance negatively affects oral bioavailability.
When the dose of the active substance does not dissolve in the approximately 250 mL of liquid that is present in the gastrointestinal lumen (a high dose number), the solid fraction of the substance will be unable to permeate across the intestinal mucosa and, consequently, will be eliminated in the faeces. Newly discovered active substances are often very poorly soluble in water, which requires unique formulations in order to achieve therapeutically relevant drug concentrations after oral administration. In [5] the selection of formulations for substances with poor water solubility is illustrated.
For the dissolution properties of the active substance, the volume of liquid available at the site of administration is important. As outlined in Table 17.1, these volumes can vary significantly depending on the administration route.
Table 17.1
Estimated physiologically available volumes
Administration route | Liquid | Volume (mL) |
|---|---|---|
Oral | Intestinal volume/glass of water | 250 |
Rectal | Volume rectum | 3 |
Transdermal | Patch material | 0.2 |
Buccal/sublingual | Saliva | 0.9 |
Vaginal | Vaginal mucus | 1.2 |
Nasal | Nose drops | 0.2 |
A dose of a poorly soluble substance may be able to dissolve in the intestinal volume (250 mL). However, the limited volume of only 200 microlitres of fluid available in the nasal cavity will be insufficient to allow complete dissolution of the same dose when administered as a nasal formulation. It must be noted therefore that literature most often classifies substances into a certain BCS class based on the oral application of the highest available dose. When the oral application concerns class l characteristics this would not necessarily hold for the nasal use since this may be considered as class II, as indicated above.
For most pharmacological targets, a medicine has to permeate across a biological membrane (absorption) after dissolution is completed in order to exert its desired therapeutic effect. In general, absorption characteristics differ considerably between the various administration routes, mainly due to anatomical features of individual barriers. For example, the skin differs to a large extent from the barrier properties of the intestinal mucosa. Physiologically, the skin provides protection and, consequently, only lipophilic substances can passively permeate this barrier. In contrast, the gastrointestinal tract is specialised for absorption of nutrients and water to maintain a viable organism. To effectively accomplish this physiological task, a combination of active and passive transport mechanisms are available for absorption across the gastrointestinal barrier.
Bipolar depression case study #2: modification of administration route
For the acute phase, a fast acting, sedative preparation is required, such as an injection or fast acting mouth spray. However, such preparations may have not only a different absorption profile, but also a different metabolic profile (e.g. because of enzymatic saturation). Thus, additional clinical research is required.
Rectal Absorption of Oxcarbazepine [6]
A mentally disabled young woman with status epilepticus was admitted to the hospital. The parents had misunderstood that the oral oxcarbazepine (1,600 mg/day) should not be terminated, after which the seizure occurred. The clinical situation did not allow for administration of oxcarbazepine per feeding tube. Instead, oxcarbazepine was given rectally as lipid-based suppositories (three times a day 400 mg). After 40 h, still no therapeutic blood level had been obtained, and thus a feeding tube was inserted after all, making enteral therapy possible. Adequate levels were obtained within 1–2 days.
The pharmacist should have anticipated the biopharmaceutical consequences of the physico-chemical properties of oxcarbazepine. The drug is classified as a Class II substance for oral application. Logically, lack of adequate solubility is even more evident for the rectal administration as the volume of rectal fluid is limited (see Table 17.1). With an aqueous solubility of approximately 300 mg/L, the solubility of the substance in the lipophilic base of the suppositories would certainly not be higher than 9.5 mg/mL (being a direct consequence of the value of the log P = 1.5 of oxcarbamazepine). This means that oxcarbazepine is not dissolved in the lipid but dispersed as crystals, which settle from the molten suppository once introduced in the rectal cavity. The amount of rectal liquid is limited and therefore a saturated solution will exist which involves only less than 1 mg dissolved oxcarbamazepine. Low solubility yields a low concentration and hence a low driving force for diffusion to occur. As a consequence, the rate of absorption is relatively low. This slow release may lead to hardly any uptake, due to defecation within several hours after insertion.
17.4.2 Permeability; Membrane Passage
Transfer of an active substance across a biological membrane is influenced by different physico-chemical drug properties, which are combined in Lipinski’s ([7] ‘Rule-of-5’. This rule predicts poor absorption of an orally administered active substance when it:
Has a molecular weight > 500 Da
Is lipophilic (octanol-water partition coefficient, log P > 5)
Has the ability to form multiple hydrogen bonds
Lipinski’s Rule-of-5 is not a physical law but allows reasonable prediction of membrane permeation properties of molecules that are mainly absorbed by passive diffusion. Many exceptions to this rule are reported in the literature, mainly for substances that bind to membrane transporters, including vitamins and antibiotics.
17.4.3 Liver Passage
Before an active substance enters the systemic circulation, it may be subjected to metabolic processes in the intestinal wall and the liver (usually to a much larger extent). This first-pass effect may diminish the availability of the active substance even when all other biopharmaceutical characteristics are adequate. See also Sect. 16.1.8.
17.5 Formulation
Before initiating the design of a formulation and method of preparation, additional physico-chemical properties of the active substance should be defined such as particle size and particle size distribution, salt, polymorphism, aqueous solubility in dependence on pH, hygroscopicity, melting point, sublimation behaviour, water of crystallisation, dehydration temperature. The properties of the raw material are also relevant to the physical and chemical stability and the compatibility with the excipients and packaging. Furthermore, compatibility with other active substances has to be investigated when the new substance is to be administered through the same infusion line.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


