CHAPTER 1 Principles of Cell Function
The human body is composed of billions of cells, each with a distinct function. Despite this diversity in cell function, all cells share certain common elements and functions. This chapter provides an overview of these common elements and focuses on the important function of transport of molecules and water into and out of the cell across its plasma membrane.
OVERVIEW OF EUKARYOTIC CELLS
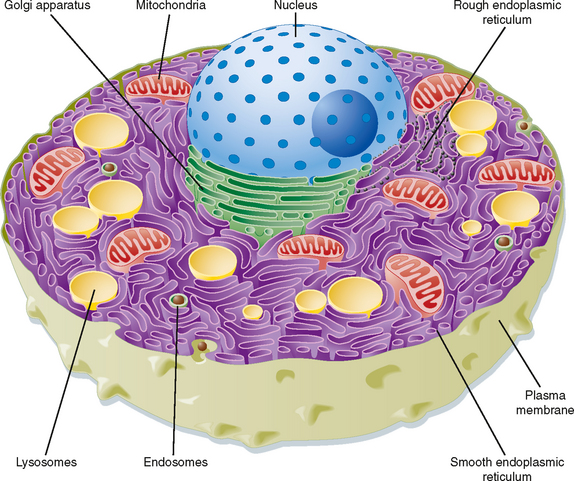
Eukaryotic cells are distinguished by the presence of a membrane-delimited nucleus. With the exception of mature human red blood cells, all cells within the body contain a nucleus. The cell is therefore effectively divided into two compartments: the nucleus and the cytoplasm. The cytoplasm is an aqueous solution containing numerous organic molecules, ions, cytoskeletal elements, and a number of organelles. A brief description of the components of a typical eukaryotic cell follows (Fig. 1-1). Readers who desire a more in-depth presentation of this material are encouraged to consult one of the many cellular and molecular biology textbooks currently available.
Smooth Endoplasmic Reticulum
The smooth endoplasmic reticulum (sER) is devoid of ribosomes and therefore appears “smooth” on electron micrographs. It is a site where many substances are modified and detoxified (e.g., pesticides). Hydrophobic molecules can be converted to water-soluble molecules in the sER, thus facilitating their excretion from the body by the liver and kidneys. The sER is also the site for the synthesis of fats and lipids. For example, the cells of the adrenal gland that secrete the steroid hormone cortisol have an extensive sER. Similarly, the cells within the ovaries and testes that secrete estrogens and testosterone have a well-developed sER. In skeletal and cardiac muscle, the sER, which is called the sarcoplasmic reticulum in these cells, serves to sequester Ca++. Thus, it plays an important role in controlling contraction.
Cytoskeleton
The cytoskeleton of the cell consists of actin filaments (also called microfilaments), intermediate filaments, and microtubules. Actin filaments in muscle cells are critical components of the contractile apparatus. In other cells they are involved in locomotion (e.g., macrophages). Actin also makes up the core of microvilli and links the interior of the cell to adjacent cells through some cell junctions (e.g., zonula adherens and zonula occludens). There are several different classes of intermediate filaments, and they can vary by cell type. For example, keratin filaments are found in epithelial cells, whereas neurofilaments are found in neurons. Intermediate filaments are primarily structural in function and can link the interior of the cell to adjacent cells and the surrounding extracellular matrix through desmosomes and hemidesmosomes, respectively. Microtubules serve multiple functions within the cell, including intracellular transport of vesicles, chromosome movement during mitosis and meiosis, and movement of cilia and flagella (e.g., tail of spermatozoa). They are formed from α- and β-tubulin dimers and change length by either adding or removing tubulin dimers. In general, a microtubule-organizing center exists near the cell’s nucleus, and microtubules grow out from this center toward the periphery of the cell. As noted, microtubules can move intracellular vesicles within the cell (e.g., transport of neurotransmitter-containing vesicles from the cell body of the neuron down the axon); such movement is driven by motor proteins. One motor protein, kinesin, drives transport from the center of the cell toward the periphery, whereas another motor protein, dynein, drives movement in the opposite direction. Dynein is the motor protein that drives the movement of both cilia and flagella.
THE PLASMA MEMBRANE
Kartagener’s syndrome is an autosomal recessive disorder in which dynein is missing in cilia and, in males, the flagella of sperm. Accordingly, males with this syndrome are infertile. Because the cilia of the epithelial cells that line the respiratory track work to remove inhaled pathogens, a process termed mucociliary transport (see Chapter 20), both men and women with this syndrome are susceptible to repeated lung infections.
Structure and Composition
The plasma membrane of eukaryotic cells consists of a 5-nm-thick lipid bilayer with associated proteins (Fig. 1-2). Some of the membrane-associated proteins are integrated into the lipid bilayer, whereas others are more loosely attached to the inner and outer surfaces of the membrane, often by binding to the integral membrane proteins. Because the lipids and proteins can diffuse within the plane of the membrane and the appearance of the membrane varies regionally as a result of the presence of different membrane proteins, this depiction of the structure of the plasma membrane is often termed the fluid mosaic model.
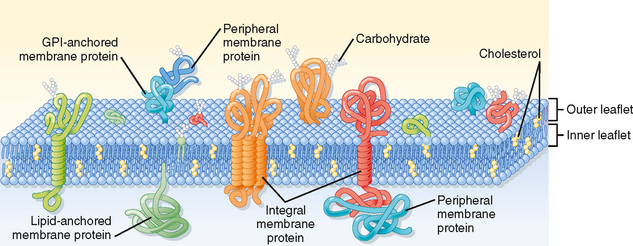
Figure 1-2 Schematic diagram of the cell plasma membrane. Not shown are lipid rafts. See text for details.
(Modified from Figure 12-3 in Cooper GM: The Cell—A Molecular Approach, 2nd ed. Washington DC, Sinauer, 2000.)
Membrane Lipids
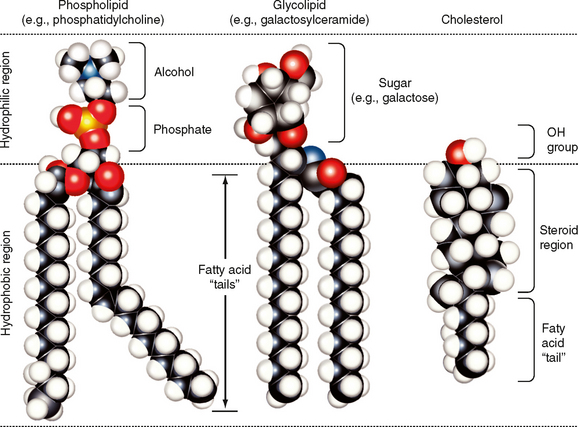
The major lipids of the plasma membrane are phospholipids or phosphoglycerides. Phospholipids are amphipathic molecules that contain a charged (or polar) hydrophilic head and two (nonpolar) hydrophobic fatty acyl chains (Fig. 1-3). The amphipathic nature of the phospholipid molecule is critical for formation of the bilayer, with the hydrophobic fatty acyl chains forming the core of the bilayer and the polar head groups exposed on the surface.
The majority of membrane phospholipids have a glycerol backbone to which are attached the fatty acyl chains, as well as an alcohol linked to glycerol via a phosphate group. The common alcohols are choline, ethanolamine, serine, inositol, and glycerol. Another important phospholipid, sphingomyelin, has the amino alcohol sphingosine as its backbone instead of glycerol. Table 1-1 lists these common phospholipids. The fatty acyl chains are usually 14 to 20 carbons in length and may be saturated or unsaturated (i.e., contain one or more double bonds).
Table 1-1 Plasma Membrane Lipids
| Phospholipid | Leaflet Location |
|---|---|
| Phosphatidylcholine | Outer |
| Sphingomyelin | Outer |
| Phosphatidylethanolamine | Inner |
| Phosphatidylserine | Inner |
| Phosphatidylinositol* | Inner |
* Involved in signal transduction.
The phospholipid composition of the membrane varies among different cell types and even between the bilayer leaflets. As summarized in Table 1-1, phosphatidylcholine and sphingomyelin are found predominantly in the outer leaflet of the membrane, whereas phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol are found in the inner leaflet. As described in detail in Chapter 3, phosphatidylinositol plays an important role in signal transduction, and its location in the inner leaflet of the membrane facilitates this signaling role.
The sterol molecule cholesterol is also a critical component of the bilayer (Fig. 1-3). It is found in both leaflets and serves to stabilize the membrane at normal body temperature (37° C). Cholesterol can represent as much as 50% of the lipids found in the membrane. Another minor lipid component of the plasma membrane is glycolipid. These lipids, as their name indicates, contain two fatty acyl chains linked to polar head groups that consist of carbohydrates (Fig. 1-3). As discussed later, one glycolipid, glycosylphosphatidylinositol (GPI), plays an important role in anchoring proteins to the outer leaflet of the membrane. Both cholesterol and glycolipids, like the phospholipids, are amphipathic and orient with their polar groups on the outer surface of the leaflet in which they are located. Their hydrophobic portions are thus located within the interior of the bilayer.
The lipid bilayer is not a static structure. Lipids can freely diffuse within the plane of the membrane. The fluidity of the membrane is determined by temperature and by its lipid composition. As temperature increases, the membrane becomes more fluid. The presence of unsaturated fatty acyl chains in phospholipids and glycolipids also increases membrane fluidity. If a fatty acyl chain is unsaturated, the presence of a double bond introduces a “kink” in the molecule (Fig. 1-3). This kink prevents the molecule from closely associating with surrounding lipids, and as a result membrane fluidity is increased. Some membranes contain lipids (e.g., sphingomyelin and cholesterol) that aggregate into what are called lipid rafts. These lipid rafts often have specific proteins associated with them and diffuse in the plane of the membrane as a discrete unit. Lipid rafts appear to serve a number of functions. One important function of these rafts is to segregate signaling mechanisms and molecules.
Membrane Proteins
As much as 50% of the membrane is composed of protein. These membrane proteins are classified as either integral, lipid anchored, or peripheral (Fig. 1-2).
There is a superfamily of membrane proteins that serve as receptors for many hormones, neurotransmitters, and numerous drugs. These receptors are coupled to heterotrimeric G proteins and are termed G protein–coupled receptors (see Chapter 3). These proteins span the membrane with seven α-helical domains. The extracellular portion of the protein contains the ligand binding site, whereas the cytoplasmic portion binds to the G protein. This superfamily of membrane proteins makes up the third largest family of genes in humans. Nearly half of all nonantibiotic prescription drugs are targeted toward G protein–coupled receptors.
MECHANISMS OF MEMBRANE TRANSPORT
Membrane Transport Proteins
Table 1-2 lists the major classes of membrane transport proteins, their mode of transport, and the rate at which they transport molecules or ions across the membrane.
Table 1-2 Major Classes of Plasma Membrane Transporters
| Class | Transport Mode | Transport Rate |
|---|---|---|
| Water channel | Gated* | Up to 109 molecules/sec |
| Ion channel | Gated | 106-108 molecules/sec |
| Solute carrier | Cycle | 102-104 molecules/sec |
| ATP dependent | Cycle | 102-104 molecules/sec |
* Water channels (i.e., aquaporins) may be continuously open and thus function similar to a pore, which is not gated (e.g., the porins found in the outer membrane of mitochondria). However, the permeability of a water channel can be modified and is therefore listed as gated.
Water Channels
Water channels, or aquaporins (AQPs), are the main route for water movement into and out of the cell. They are widely distributed throughout the body, although different isoforms are found in different cell types. To date, 12 AQPs have been identified. The amount of H2O that can enter or leave the cell via AQPs can be regulated by altering the number of AQPs in the membrane or by changing their permeability (i.e., gating). Changes in pH have been identified as one factor that can modulate the permeability of AQPs.
Ion Channels
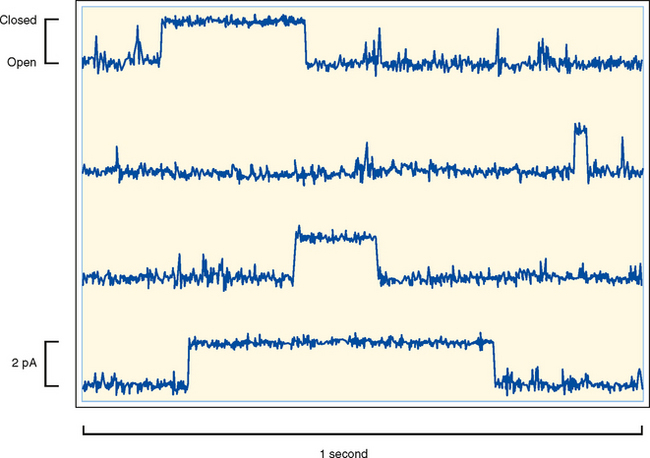
Ion channels are found in all cells and are especially important for the function of excitable cells (e.g., neurons and muscle cells). Ion channels are classified by their selectivity (i.e., the ions that pass through the channel). At one extreme, they can be highly selective by allowing only a specific ion through. At the other extreme, they may be nonselective and allow all or a group of cations or anions through. Channels are also characterized by their conductance, which is typically expressed in picosiemens (pS). The range in conductance is considerable, with some channels having a conductance of only 1 to 2 pS and others having a conductance of greater than 100 pS. For some channels, conductance varies depending on which direction the ion is moving. For example, if the channel has greater conductance when ions are moving into the cell versus out of the cell, the channel is said to be an inward rectifier. Finally, ion channels can be classified by their mechanism of gating. As illustrated in Figure 1-4, ion channels fluctuate between an open state or a closed state, a process called gating. Factors that can control gating include membrane voltage, extracellular agonists or antagonists (e.g., acetylcholine is an extracellular agonist that controls the gating of a cation-selective channel in the motor end plate of skeletal muscle cells–see Chapter 12), intracellular messengers (e.g., Ca++, ATP, cGMP), and mechanical stretch of the plasma membrane. Trans-membrane ion flux can be regulated by changing the number of channels in the membrane or by gating of the channels.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree