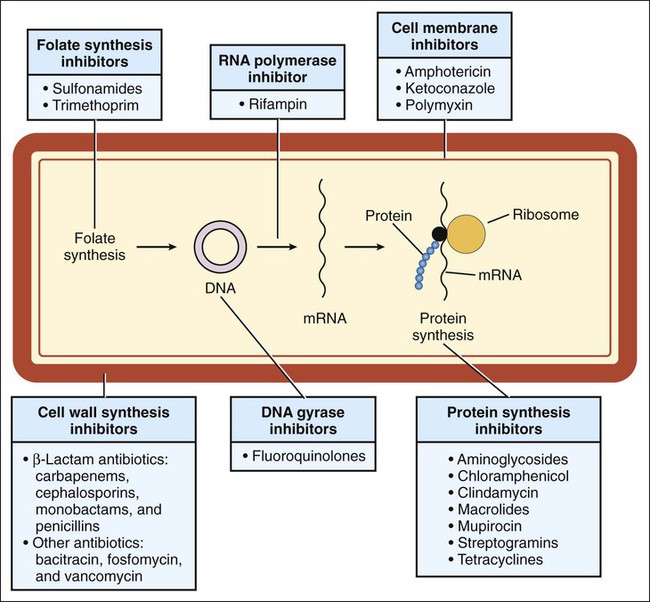
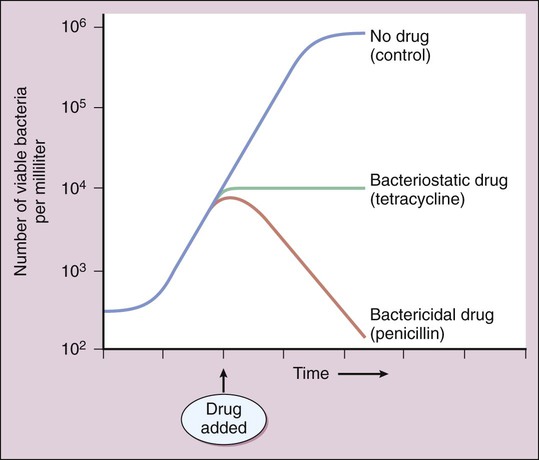
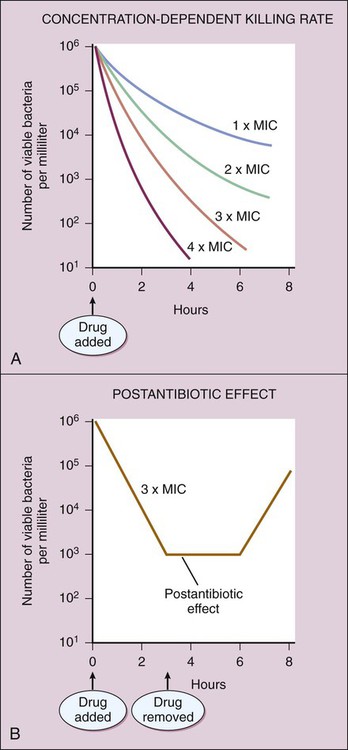
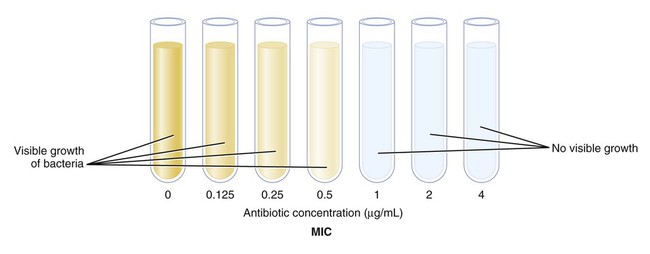
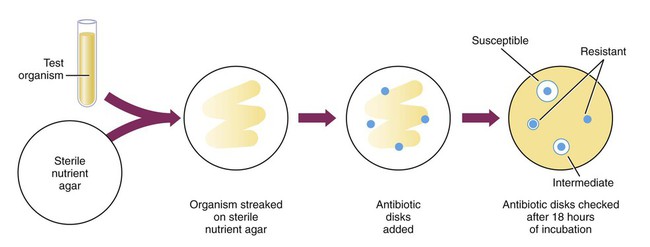
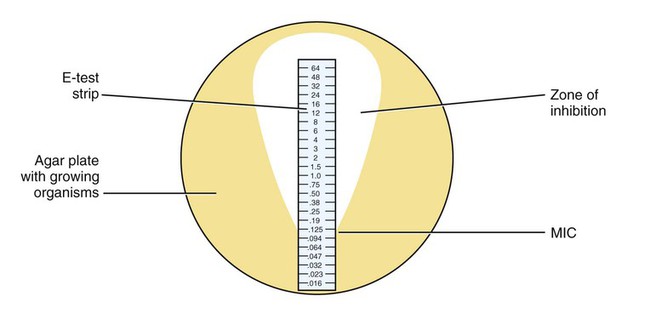
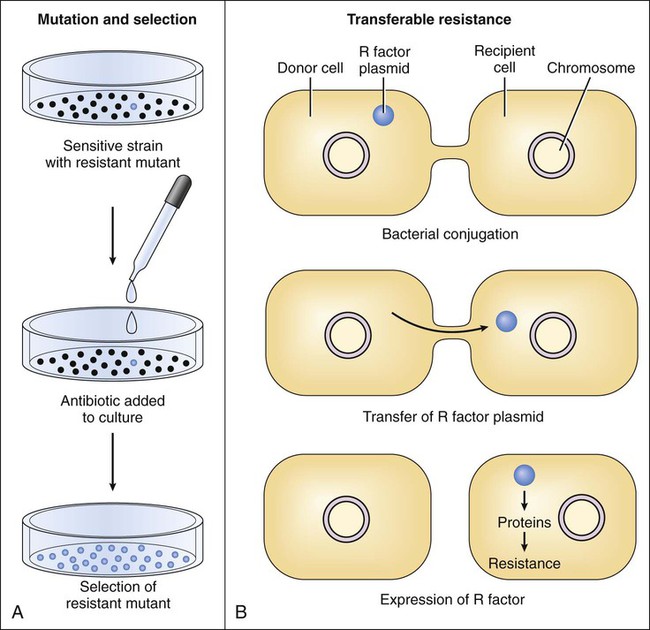
Chemotherapy is defined as the use of drugs to eradicate pathogenic organisms or neoplastic cells in the treatment of infectious diseases or cancer. Chemotherapy is based on the principle of selective toxicity. According to this principle, a chemotherapeutic drug inhibits a vital function of invading organisms or neoplastic cells that differs qualitatively or quantitatively from functions of host cells. The chemotherapeutic drugs include antimicrobial drugs (introduced in this chapter and discussed further in Chapters 38 to 43), antiparasitic drugs (discussed in Chapter 44), and antineoplastic drugs (discussed in Chapter 45). Antimicrobial drugs are usually classified on the basis of their site and mechanism of action and are subclassified on the basis of their chemical structure. The antimicrobial drugs include cell wall synthesis inhibitors, protein synthesis inhibitors, metabolic and nucleic acid inhibitors, and cell membrane inhibitors. The sites of action of these drugs are depicted in (Fig. 37-1), and their mechanisms of action, pharmacologic properties, and clinical uses are described in subsequent chapters. A bactericidal drug kills sensitive organisms so that the number of viable organisms falls rapidly after exposure to the drug (Fig. 37-2). In contrast, a bacteriostatic drug inhibits the growth of bacteria but does not kill them. For this reason, the number of bacteria remains relatively constant in the presence of a bacteriostatic drug, and immunologic mechanisms are required to eliminate organisms during treatment of an infection with this type of drug. (The same principle applies to a drug that kills or inhibits the growth of fungi and is referred to as a fungicidal drug or a fungistatic drug, respectively.) An example of a CDKR is shown in Figure 37-3A. Some aminoglycosides (e.g., tobramycin) and some fluoroquinolones (e.g., ciprofloxacin) exhibit a CDKR against a large group of gram-negative bacteria, including Pseudomonas aeruginosa and members of the family Enterobacteriaceae. In contrast, penicillins and other β-lactam antibiotics usually do not exhibit a CDKR. After an antibacterial drug is removed from a bacterial culture, evidence of a persistent effect on bacterial growth may exist. This effect (see Fig. 37-3B) is the PAE. Most bactericidal antibiotics exhibit a PAE against susceptible pathogens. For example, penicillins show a PAE against gram-positive cocci, and aminoglycosides show a PAE against gram-negative bacilli. Because aminoglycosides exhibit both a CDKR and a PAE, treatment regimens have been developed in which the entire daily dose of an aminoglycoside is given at one time. Theoretically, the high rate of bacterial killing produced by these regimens would more rapidly eliminate bacteria, and the PAE would prevent any remaining bacteria from replicating for several hours after the drug has been eliminated from the body. Microbial sensitivity to drugs can be determined by various means, including the broth dilution test, the disk diffusion method (Kirby-Bauer test), and the E-test method. These laboratory procedures are described in Box 37-1. Microbes can spontaneously mutate to a form that is resistant to a particular antimicrobial drug. These mutations occur at a relatively constant rate, such as in 1 in 1012 organisms per unit of time. If the organisms are exposed to an antimicrobial drug during this time period, the sensitive organisms may be eradicated, enabling the resistant mutant to multiply and become the dominant strain (Fig. 37-4A). Transferable resistance usually results from bacterial conjugation and the transfer of plasmids (extrachromosomal DNA) that confer drug resistance (see Fig. 37-4B). Transferable resistance, however, can also be mediated by transformation (uptake of naked DNA) or transduction (transfer of bacterial DNA by a bacteriophage). Bacterial conjugation enables a bacterium to donate a plasmid containing genes that encode proteins responsible for resistance to an antibiotic. These genes are called resistance factors. The resistance factors can be transferred both within a particular species and between different species, so they often confer multidrug resistance. The various species need not all be present during the period in which the antibiotic is administered. Studies have shown that resident microflora of the human body can serve as reservoirs for resistance genes, allowing the transfer of these genes to organisms that later invade and colonize the host.
Principles of Antimicrobial Chemotherapy
Overview
Classification of Antimicrobial Drugs
Antimicrobial Activity
Bactericidal or Bacteriostatic Effect
Concentration- and Time-Dependent Effects
Microbial Sensitivity and Resistance
Laboratory Tests for Microbial Sensitivity
Microbial Resistance to Drugs
Origin of Resistance
Mutation and Selection.
Transferable Resistance.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Principles of Antimicrobial Chemotherapy
Only gold members can continue reading. Log In or Register to continue