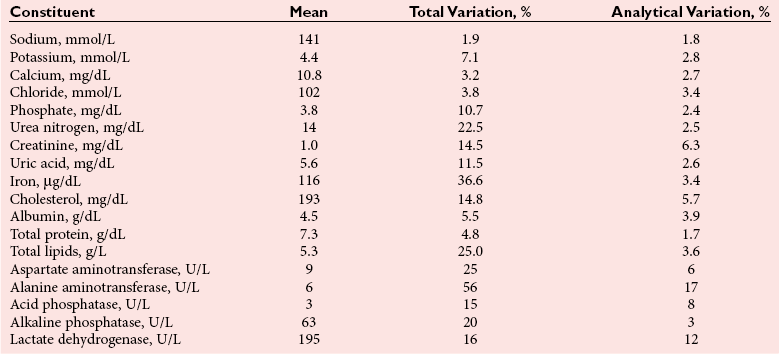
Chapter 6 The human body is composed of many different compounds and elements; the concentration or activity of these analytes in body fluids may reflect an individual’s health or pathophysiological state. Many factors other than disease may affect the concentration or activity of these analytes.31,49 If pertinent for the requested tests, the phlebotomist should verify that the patient is fasting. Ideally, a patient should have remained in the same position for 30 minutes before a specimen is collected, and in the same position as likely to be appropriate for the next specimen to be collected (e.g., supine if an inpatient, sitting if an outpatient). A tourniquet should be used to facilitate location of a vein for venipuncture, but application for longer than 1 minute begins to induce hemoconcentration. An appropriately sized needle should be used to lessen the possibility of hemolysis. Other precautions must also be taken to prevent hemolysis—no shaking of tubes or vigorous mixing of blood or puncturing of skin before the alcohol used to clean the skin has evaporated.105 Hemolysis may lead to false results through leakage of analytes from erythrocytes or through interference with certain photometric methods.104 The extent of the interference is typically related to the degree of hemolysis. Use of an evacuated blood tube system to collect blood is preferred to use of a syringe to minimize hemolysis, and blood collected into one type of tube should never be transferred into another tube. Each laboratory must define which types of specimens are appropriate for the analytical methods that it uses. Generally, plasma allows more rapid processing of specimens for chemistry tests, but anticoagulants may interfere with some analytical methods.104 However, serum concentrations of potassium and phosphate may be as much as 8.4% and 7.0% higher, respectively, than in plasma, and other analytes may be affected to a lesser extent.58 The composition of blood from different vascular locations (e.g., capillary, artery, vein) may be slightly different, so consistent use of the same source of blood is desirable.105 Once specimens have been collected, they should be transported rapidly to a laboratory for testing. If a blood collection site is distant from a laboratory, specimens should be collected into evacuated blood tubes containing a thixotropic polymer gel and should be centrifuged on site. The gel forms an effective barrier between the separated serum or plasma and cells, so no leakage of cellular constituents occurs into the plasma or serum above the gel. Centrifugation of specimens should be done within 2 hours of blood collection, and within the laboratory, if specimens cannot be tested in a timely manner, they must be held under appropriate storage conditions—at room temperature, refrigerated, or frozen, depending on the analyte—until testing takes place.105 The typical pressure at the arterial end of a capillary is 24 mm Hg (3.2 kPa), and at the venous end 10 mm Hg (1.3 kPa), although this varies with the distance of the capillary from the heart.74 Transfer of fluid and solute across a capillary wall depends on a complex interaction of hydrostatic and osmotic pressures of capillary and interstitial fluids. Fluid moves into the interstitial space at the arteriolar end of the capillary and returns to the capillary at the venular end. A greater volume of fluid leaves the capillary at the arteriolar end than is returned to the venous end. Excess fluid drains into the lymphatic system. When an individual lies down, fluid return to the capillaries is increased, because capillary pressure is reduced. The volume of fluid returning to capillaries progressively declines when an individual is recumbent for a long time. Heart rate and systolic and diastolic blood pressures are greater in the upright than in the recumbent individual. Change in posture from lying to standing increases the secretion of catecholamines, aldosterone, angiotensin II, renin, and antidiuretic hormone (vasopressin). Epinephrine and norepinephrine concentrations in plasma may double within 10 minutes, but no change in their urinary excretion is noted. The increase in plasma aldosterone and plasma renin activity is slower, but their concentrations may still double within 1 hour. Concentrations of other hormones may also increase as a result of the relative hemoconcentration induced by standing. Typically, a 5 to 15% increase in the concentrations of most cellular elements and protein-bound molecules is also noted with a change from lying to an erect position. Substantial changes also take place with a change from lying to a sitting position, or from standing to a supine or sitting position.16 Reduction of the extracellular fluid volume with standing reduces the renal blood flow and causes a reduction in the glomerular filtration rate (GFR) and in urine production. Changes are apparent in 1 hour. Within 2 hours of becoming recumbent, an individual’s hemoglobin and hematocrit may decrease by as much as 6.5% as the result of hypervolemia. This is associated with a reduction in the concentration of plasma protein on the order of 8% and of protein-bound constituents.38 Although postural changes affect urinary sodium excretion, its plasma concentration is only slightly affected. Urinary excretion of sodium and lithium (used to treat some forms of schizophrenia) is reduced in response to increased aldosterone secretion, but the normal diurnal variation persists.41 When an individual stands, his urinary pH decreases and excretion of bicarbonate is reduced as hydrogen ions are exchanged for sodium. Excretion of protein is reduced in most individuals with reduction of the glomerular filtration rate that occurs with standing. Orthostatic proteinuria is a condition in which protein is present when individuals are standing but is essentially absent when they are recumbent. This phenomenon may be caused by increased glomerular permeability from increased venous pressure. The incidence of orthostatic proteinuria is probably less than 5%. Changes in concentration of proteins and protein-bound constituents in serum with postural changes are greater in hypertensive patients than in normotensive patients, in individuals with a low plasma protein concentration than in those with a normal concentration, and in the elderly compared with the young.25,26 Most of the plasma oncotic pressure is attributable to albumin because of its high concentration, so that protein malnutrition—with its associated reduction in plasma albumin concentration—reduces the retention of fluid within the capillaries. Conversely, the impact of postural changes is less in individuals with abnormally high concentrations of protein, such as those with a monoclonal gammopathy (multiple myeloma). In general, the concentrations of freely diffusible constituents with molecular weights of less than 5000 Da are unaffected by postural changes. However, a significant increase in potassium (≈0.2 to 0.3 mmol/L) occurs after an individual stands for 30 minutes.27 This increase in K+ has been attributed to the release of intracellular potassium from muscle. Changes in the concentration of some major serum constituents with changes in posture are listed in Table 6-1. TABLE 6-1 Change in Concentration of Serum Constituents With Change from Lying to Standing From Felding P, Tryding N, Hyltoft Petersen P, Hørder M. Effects of posture on concentrations of blood constituents in healthy adults: practical application of blood specimen collection procedures recommended by the Scandinavian Committee on Reference Values. Scand J Clin Lab Invest 1980;40:615-21. Plasma and extracellular fluid volumes decrease within a few days of the start of bed rest. Consequently, the blood hematocrit may increase by as much as 10% within 4 days. Usually a slight reduction in total body water is noted, but with 2 weeks’ bed rest the plasma volume reverts to its pre-bed rest value.38 Prolonged bed rest is associated with increased urinary nitrogen excretion, which increases by up to 15% after 2 weeks. Calcium excretion steadily increases up to 7 weeks of rest, increasing by a maximum of about 60%. Excretion of sodium, potassium, phosphate, and sulfate is also increased but to a much smaller extent; hydrogen ion excretion is reduced, and this is presumably caused by decreased metabolism of skeletal muscle.22 The amplitude of circadian variation of plasma cortisol is reduced by prolonged immobilization, and urinary excretion of catecholamines may be reduced to one third of the concentration in an active individual. Vanillylmandelic acid (VMA) excretion is reduced by one fourth after 2 to 3 weeks of bed rest. In considering the effects of exercise, the nature and extent of the exercise should be taken into account.86 Static or isometric exercise, usually of short duration but of high intensity, uses previously stored ATP and creatine phosphate, whereas more prolonged exercise must use ATP generated by normal metabolic pathways. Changes in concentrations of analytes that result from exercise are largely due to shifts of fluid between intravascular and interstitial compartments and changes in hormone concentrations stimulated by the change in activity and by the loss of fluid due to sweating. Plasma concentrations of β-endorphin and catecholamines may more than double within a minute of initiation of strenuous exercise. Hemoconcentration, affecting high molecular weight constituents, follows strenuous exercise. The physical fitness of an individual may also affect the extent of change in the concentration of a constituent; the length of time after exercise when a specimen was collected also influences the concentrations of measured analytes. Such factors account for sometimes conflicting reports in the literature. With moderate exercise, the provoked stress response causes an increase in blood glucose, which stimulates insulin secretion. The arteriovenous difference in glucose concentration is increased more than 5-fold from about 14 mg/dL (0.8 mmol/L) at rest, depending on the duration and intensity of exercise in association with greater tissue demand for glucose.97 Plasma pyruvate and lactate are increased by increased metabolic activity of skeletal muscle. Even mild exercise may increase the plasma lactate twofold. Arterial pH and PCO2 are reduced by exercise. Reduced renal blood flow causes a slight increase in the serum creatinine concentration. Competition for renal excretion between urate, lactate, and products of increased tissue catabolism causes the plasma urate concentration to increase. Exercise causes a reduction in cellular ATP, which increases cellular permeability. Increased permeability causes slight increases in the serum activities of enzymes originating from skeletal muscle, such as AST, LD, CK, and aldolase.94 The increase in CK is largely attributable to its CK-MM isoform, although small increases in CK-MB may also be observed. The increase in enzyme activity tends to be greater in unfit than in fit individuals. Performing normal daily activities over 4 hours may increase serum CK activity by as much as 50% in some healthy individuals.48 Mild exercise produces a slight decrease in serum cholesterol and triglyceride concentrations that may persist for several days. Those who walk for about 4 hours each week have an average cholesterol concentration 5% lower and HDL concentration 3.4% higher than inactive individuals. In general, the effects of strenuous exercise are exaggerations of those occurring with mild exercise. Thus hypoglycemia and increased glucose tolerance may occur. Plasma lactate may be increased tenfold during exercise but soon returns to normal in fit individuals. Severe exercise increases the concentration of plasma proteins owing to an influx of protein from interstitial spaces, which occurs after an initial loss of both fluid and protein through the capillaries. Plasma concentrations of total proteins increase by about 9% and renal glomerular permeability increases, leading to increased proteinuria.75 Plasma fibrinolytic activity is also increased. Strenuous exercise may more than double CK activity, but the activity of enzymes with primarily liver or kidney origin is little changed, although hepatic and renal blood flow is reduced. Strenuous exercise for 10 minutes increases plasma renin activity by 400%. Cortisol secretion is stimulated, and the normal diurnal variation may be abolished.21 Urinary free cortisol excretion and plasma concentrations of cortisol, aldosterone, growth hormone (somatotropin), and prolactin are also increased by exercise. Plasma insulin concentration is decreased by exercise. Strenuous exercise increases both plasma and urinary concentrations of catecholamines. Changes in concentrations of cortisol and other stress-stimulated increased hormone concentrations are presumed to release leukocytes, primarily neutrophils, from the bone marrow into the peripheral circulation. Following strenuous exercise, the leukocyte count has been observed to increase to about 25,000 cells/µL.41 Some representative changes in concentration or activity of serum constituents induced by exercise are listed in Table 6-2. TABLE 6-2 Effects of Strenuous Exercise on Selected Serum Constituents* *Changes were determined 15 minutes after conclusion of 20 minutes of exercise. From Statland BE, Winkel P, Bokelund H. Factors contributing to variation of serum constituents in healthy subjects. In: Siest G, ed. Organisation des laboratoires: biologie perspective. Paris: L’Expansion Scientifique Francaise, 1975:717-50. Athletes generally have a higher serum activity of enzymes of skeletal muscular origin at rest than do nonathletes. However, the response of these enzymes to exercise is less in athletes than in other individuals. Reduced release of enzymes from skeletal muscle in well-trained individuals has been attributed to an increase in the number and size of mitochondria, allowing the muscle to better metabolize glucose, fatty acids, and ketone bodies. The proportion of CK that is CK-MB is much greater in trained than in untrained individuals.14 Serum concentrations of urea, urate, creatinine, and thyroxine are higher in athletes than in comparable untrained individuals. Urinary excretion of creatinine is also increased. These changes are probably related to increased muscle mass and greater turnover of muscle mass in athletes. Many constituents of body fluids exhibit cyclical variation throughout the day.95,100 Factors contributing to such variation include posture, activity, food ingestion, stress, daylight or darkness, and sleep or wakefulness. The cyclical pattern tends to be similar among individuals who work during the day and sleep at night, although it is different in night workers. These cyclical variations may be quite large; therefore the timing of specimen collection must be strictly controlled. For example, the concentration of serum iron may increase by as much as 50% from 0800 to 1400, and that of cortisol by a similar amount between 0800 and 1600. Serum potassium has been reported to decline from 5.4 mmol/L at 0800 to 4.3 mmol/L at 1400.88,89 The typical total variation in several commonly measured serum constituents over 6 hours is illustrated in Table 6-3. Total variation is contrasted with analytical error. TABLE 6-3 Total and Analytical Variation for Serum Tests on Specimens Obtained at 0800 and 1400* *11 male subjects, age 21 to 27 years, studied at 0800, 1100, and 1400. From Winkel P, Statland BE, Bokelund H. The effects of time of venipuncture on variation of serum constituents. Am J Clin Pathol 1975;64:433-47. Copyright © 1975 by the American Society of Clinical Pathologists. Reprinted with permission. Hormones are secreted in bursts, and this, together with the cyclical variation to which most hormones are subject, may make it very difficult to interpret their plasma concentrations properly.99 Corticotropin secretion is influenced by cortisol-like steroids, but it is also affected by posture and by light, darkness, and stress. Its secretion is increased threefold to fivefold from its minimum between afternoon and midnight to its maximum around waking. Growth hormone and epinephrine concentrations exhibit similar change throughout the day. Cortisol concentrations are greatest around 0600 to 0800 hours, and one study reported mean minima and maxima of 15.8 µg/dL and 111 µg/dL, respectively, at different times during the day.52 Maximum renin activity normally occurs early in the morning during sleep; its minimum occurs late in the afternoon. The plasma aldosterone concentration shows a similar pattern. GFR varies inversely with the secretion of renin, probably through constriction of renal efferent arterioles.42 GFR is least at the time of maximum renin secretion and is 20% greater in the afternoon, when renin activity is at a minimum. Excretion of 17-ketosteroids and 17-hydroxycorticosteroids is low at night and reaches a maximum about midafternoon. No circadian variation in plasma concentrations of FSH and LH is noted in men, but a 20 to 40% increase in plasma testosterone occurs during the night. Prolactin is secreted, similar to other hormones such as LH and FSH, in multiple bursts; prolactin concentration is greatest during sleep.99 The pituitary gland regulates hormone secretion primarily through negative feedback generated by increased circulating concentrations of circulating hormones. To perform a precise assessment of the concentration of a hormone, several measurements may be required. Serum TSH is at a maximum between 0200 and 0400, and is at a minimum between 1800 and 2200. The variation is on the order of 50 to 206%.52 Variations in serum thyroxine concentrations also occur, but these appear to be related to changes in the concentration of binding protein brought about by changes in posture. These variations are maximal at between 1000 and 1400. Total protein concentration may vary by as much as 10% over 24 hours, but variation in individual proteins may be even greater. Peak urinary excretion of sodium and potassium occurs about noon, whereas excretion of calcium and magnesium is greatest during the night. Urinary phosphate excretion is low at night, with the result that serum phosphate is as much as 30% higher at night than during the morning. Urinary volume and creatinine excretion are low during the night. Creatinine clearance may be reduced by up to 10% during the night. Night urine contains excess ammonia, and its titratable acidity is high.90 Blood cell concentrations are affected by circadian rhythms, with neutrophil and lymphocyte counts increasing by 61% and 67%, respectively, at their peaks from their nadir concentrations.52 Travel across several time zones affects the normal circadian rhythm. Five days is required to establish a new stable diurnal rhythm after travel across 10 time zones. Changes in laboratory test results are attributable to altered pituitary and adrenal function. Urinary excretion of catecholamines is usually increased for 2 days, and serum cortisol is reduced. During a 20-hour flight, serum glucose and triglyceride concentrations increase, while glucocorticoid secretion is stimulated. During such a prolonged flight, fluid and sodium retention occurs, but urinary excretion returns to normal after 2 days.11 Space travel is associated with a decrease in blood and plasma volumes and is further associated with increases in plasma antidiuretic hormone, atrial natriuretic peptide, growth hormone, cortisol, and corticotropin concentrations.60 In contrast, the plasma renin activity may be decreased by as much as 50%. Plasma aldosterone may also decrease but to a lesser extent. In spite of the stress of space travel, plasma concentrations of catecholamines are usually unaffected. Space travel leads to bone demineralization and a negative calcium and phosphate balance, primarily caused by increased excretion of these minerals. However, concentrations of commonly measured analytes generally do not exceed the reference range when astronauts adapt to space travel.102 Four days after the change from a normal diet to a high-protein diet, doubling of the plasma urea concentration occurs, along with an increase in its urinary excretion.9 Serum cholesterol, phosphate, urate, and ammonia concentrations are also increased. High protein intake increases both serum and urinary urea and urate. A high-fat diet, in contrast, depletes the nitrogen pool because of the requirement for excretion of ammonium ions to maintain acid-base homeostasis. A high-fat diet increases the serum concentration of triglycerides but reduces serum urate. Reduction of fat intake reduces serum lactate dehydrogenase activity. Ingestion of very different amounts of cholesterol has little effect on its serum concentration; an increase in intake of 50% may affect the serum concentration by only 5 to 10 mg/dL (0.13 to 0.26 mmol/L).54 Ingestion of monounsaturated fat instead of saturated fat reduces cholesterol and LDL-cholesterol concentrations. When polyunsaturated fat is substituted for saturated fat, the concentrations of triglycerides and HDL-cholesterol are reduced. Notwithstanding the conclusions of previous studies, a 2009 report suggests that different types of diets have a similar influence on the plasma concentrations of lipids, and that total caloric ingestion is the primary influence on an individual’s body mass and blood lipid concentrations, with total and LDL-cholesterol and triglyceride concentrations decreasing and HDL-cholesterol increasing similarly when volunteers were fed different weight-loss diets.81 When dietary carbohydrates consist mainly of starch or sucrose rather than other sugars, the serum activities of ALP and LD are increased. AST and ALT activities are also influenced by the type of sugar ingested, being higher with a sugar diet than with a starch-based diet.51 Plasma triglyceride concentration is reduced when sucrose intake is decreased. Peak glucose concentration tends to be less during a glucose tolerance test in individuals habitually ingesting a bread diet than when a high-sucrose diet is ingested. A high-carbohydrate diet decreases the serum concentrations of LDL-cholesterol, triglycerides, cholesterol, and protein. Individuals who eat many small meals throughout the day tend to have total LDL- and HDL-cholesterol concentrations that are lower than when food of the same type and amount is eaten in three meals.2 The concentration of certain plasma constituents is affected by the ingestion of a meal, with the time between ingestion of a meal and collection of blood affecting the plasma concentrations of many analytes. For example, fasting overnight for 10 to 14 hours before blood collection noticeably decreases the variability in concentration of many analytes and is seen as the optimal time for fasting around which to standardize blood collections.24 The biggest increases in serum concentration that occur after a meal are noted for glucose and triglycerides.13 The increase in ALP (mainly intestinal isoenzyme) is greater when a fatty meal is ingested and is influenced by the blood group of the individual and the substrate used for the enzyme assay. Activities of alanine and aspartate aminotransferases may increase by 10 to 20% following a meal.49 In addition, lipemia following a meal may affect some analytical methods used to measure serum constituents. Ultracentrifugation or the use of serum blanks can reduce the adverse analytical effects of lipemia. The effects of a meal may be long lasting. Thus ingestion of a protein-rich meal in the evening may cause increases in concentration of serum urea nitrogen, phosphorus, and urate that are still apparent 12 hours later.1 Nevertheless, these changes may be less than the typical intraindividual variability. Large protein meals at lunch or in the evening increase serum cholesterol and growth hormone concentrations for at least 1 hour after a meal. The effect of carbohydrate meals on blood composition is less than that of protein meals. No change in the cortisol concentration is noted when breakfast is taken, probably because cortisol completely occupies all cortisol binding sites on its binding protein in the early morning. Glucagon and insulin secretions are stimulated by a protein meal, and insulin is also stimulated by carbohydrate meals. The effects of ingestion of a 700-kcal (2.93-MJ) meal on some commonly measured blood constituents are illustrated in Table 6-4. These effects differ with different meals. Thus the glucose increase is often greater and phosphate is usually decreased after a carbohydrate meal.90 TABLE 6-4 Influence of a Standard 700-kcal Meal on Serum Constituents* *Results are mean values in 200 healthy individuals. †Note that other studies have reported different changes based on different content and amounts of food. From Steinmetz J, Panek E, Sourieau F, Siest G. Influence of food intake on biological parameters. In: Siest G (ed). Reference values in human chemistry. Basel: Karger, 1973:193-200; Cohn JS, McNamara JR, Cohn SD, et al. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res 1988;29:469-79. (These investigators have reported increases in plasma triglyceride concentrations greater than 150% after a high-fat meal.) Bran: Habitual ingestion of bran, which is widely promoted to improve the concentration of lipids, impedes absorption of certain compounds, including calcium, cholesterol, and triglycerides, from the gastrointestinal tract. The concentration of calcium may be reduced by as much as 0.3 mg/dL (0.08 mmol/L) and that of triglycerides by 20 mg/dL (0.23 mmol/L), especially if triglycerides were high initially.46 Pectin and dietary fibers reduce serum apolipoprotein B and cholesterol concentrations. Food Constituents: The composition of common foods is often overlooked. Many fruits, such as bananas, and vegetables that contain 5-hydroxytryptamine (serotonin) cause increased excretion of 5-HIAA. Avocados impair glucose tolerance by affecting insulin secretion. Onions reduce plasma glucose and the insulin response to glucose. Garlic ingestion may reduce serum cholesterol concentrations by about 9%.104 Caffeine: Caffeine is contained in many beverages, including coffee, tea, and colas, and has a considerable effect on the concentration of blood constituents. Caffeine stimulates the adrenal medulla, causing increased secretion of epinephrine, reflected in a two- to threefold increase in the plasma epinephrine concentration. Excretion of the catecholamines and their metabolites is increased and a slight increase in plasma glucose concentration occurs as a result of increased gluconeogenesis with concomitant impairment of glucose tolerance.5 The adrenal cortex is also affected; plasma cortisol is increased, and this is accompanied by increased excretion of free cortisol, 11-hydroxycorticoids, and 5-HIAA. The effect of caffeine may be so great that the normal diurnal variation of plasma cortisol may be suppressed. Plasma renin activity may also be increased following caffeine ingestion. Caffeinated, but not decaffeinated, coffee causes diuresis with a transient increase in excretion of sodium and potassium. It does this by inhibiting the reabsorption of electrolytes in the ascending loop of Henle of the renal nephrons.66 Caffeine has a marked effect on lipid metabolism. Ingestion of two cups of coffee may increase the plasma free fatty acid concentration by as much as 30% and those of glycerol, total lipids, and lipoproteins to a lesser extent. Activation of triglyceride lipase causes an increase in nonesterified fatty acid concentration. Prolonged ingestion of caffeine (e.g., over several weeks) causes a slight reduction in the serum cholesterol concentration but an increase in the serum triglyceride concentration. Because the effect on plasma LDL-cholesterol and apolipoprotein B is greater in individuals drinking decaffeinated coffee than in those drinking regular coffee, the effects may be unrelated to caffeine.92 In long-standing vegetarians, the concentration of VLDL-cholesterol is reduced, typically by 12%, compared with nonvegetarians. Total lipid and phospholipid concentrations are reduced, and concentrations of cholesterol and triglycerides may be only two thirds of those in individuals on a mixed diet. Both HDL- and LDL-cholesterol concentrations are affected. In strict vegetarians, the LDL-cholesterol concentration may be 37% less and the HDL-cholesterol concentration 12% less than in nonvegetarians. The cholesterol : HDL-cholesterol ratio is decreased. Effects are less notable in individuals who have been on a vegetarian diet for only a short time. Lipid concentrations are also less in individuals who eat only a vegetable diet than in those who consume eggs and milk as well. Little difference is seen in the concentration of protein or the activities of enzymes in the serum of long-standing vegetarians and individuals on a mixed diet. A vegetarian diet does not appear to affect liver function in that liver function tests are similar in vegans and nonvegans.37 The serum creatinine concentration may be slightly reduced in vegetarians because of reduced ingestion of protein, but urinary excretion of creatinine and its clearance may be almost 40% less than in meat-eaters.61 Plasma concentrations of trace elements tend to be reduced in vegans. For example, serum copper may be reduced by 20%, selenium by 10%, and zinc by more than 10% after individuals have consumed a lactovegetarian diet for 3 months.87 Although the plasma concentration of many vitamins is increased, that of vitamin B12 may be reduced in vegetarians to a concentration approaching that observed in deficiency. An explanation for the low vitamin B12 and the high bilirubin still has to be established. Differences in the composition of serum of vegetarians and nonvegetarians are shown in Table 6-5. Urinary pH is usually higher in vegetarians than in meat-eaters as the result of reduced intake of precursors of acid metabolites. TABLE 6-5 Comparison of Blood Constituents Between Vegetarians and Nonvegetarians B, Whole blood; P, plasma; S, serum. From Gear JS, Mann JI, Thorogood M, Carter R, Jelfs R. Biochemical and haematological variables in vegetarians. Br Med J 1980;280:1415. In malnutrition, the plasma concentrations of most proteins, including total protein, albumin, prealbumin, and β-globulin, are reduced. The frequently increased concentration of γ-globulin does not fully compensate for the decrease in other proteins. Concentrations of complement C3, retinol-binding globulin, transferrin, and prealbumin decrease rapidly with the onset of malnutrition and are measured to define the severity of the condition.72 Plasma concentrations of lipoproteins are reduced, and serum cholesterol and triglycerides may be only 50% of the concentrations in healthy individuals. In spite of severe malnutrition, glucose concentration is maintained close to that in healthy individuals. However, the concentrations of serum urea nitrogen and creatinine are greatly reduced as a result of decreased skeletal mass, and creatinine clearance is decreased. Erythrocyte and plasma folate concentrations are reduced in protein-calorie malnutrition, but the serum vitamin B12 concentration is unaffected or may even be slightly increased.55 Plasma concentrations of vitamins A and E are reduced, but the extent depends on the cause and duration of the malnutrition (e.g., dietary or iatrogenic, such as bariatric surgery). The blood hemoglobin concentration is reduced, but the serum iron concentration initially is little affected by malnutrition, although decreased plasma transferrin concentrations ultimately lead to reduced iron concentrations. Withdrawal of most caloric intake has been used to treat certain cases of obesity. Such withdrawal provokes many metabolic responses. The body attempts to conserve protein at the expense of other sources of energy, such as fat. The blood glucose concentration decreases by as much as 18 mg/dL (1 mmol/L) within 3 days of the start of a fast, in spite of the body’s attempts to maintain glucose production.67 Insulin secretion is greatly reduced, whereas glucagon secretion may double in an attempt to maintain normal glucose concentration. Lipolysis and hepatic ketogenesis are stimulated. Amino acids are released from skeletal muscle, and the plasma concentration of branched-chain amino acids may increase by as much as 100% with 1 day of fasting, but the urea concentration decreases. Ketoacids and fatty acids become the principal sources of energy for muscle. This results in an accumulation of organic acids that leads to a metabolic acidosis with reduction of blood pH, PCO2, and plasma bicarbonate concentrations. In addition, the concentrations of ketone bodies (acetoacetic acid, β-hydroxybutyric acid, and acetone), fatty acids, and glycerol in serum rise considerably. When individuals are fasted for 60 hours compared with the usual 12 hours typically used in clinical practice to obtain baseline laboratory values, plasma insulin concentrations are reduced by half and those of C-peptide by more than one third. In contrast, concentrations of glucagon, epinephrine, and norepinephrine are doubled, and that of growth hormone is increased fivefold.10 The breakdown of fat leads to a transient increase in body water. Typically, however, an osmotic diuresis soon reduces the blood volume. Fasting for 6 days increases plasma concentrations of cholesterol and triglycerides but causes a decrease in HDL-cholesterol concentration.82 After individuals lived for 4 weeks on a 400-kcal diet, the concentrations of urea and triglycerides and the activity of gamma-glutamyltransferase decreased by 20 to 50%, whereas concentrations of urate, derived from nucleoprotein, and creatinine and the activity of AST increased by 20 to 40%.96 Reduced GFR and competition for excretion from lactate and ketoacids contribute to the increased urate concentration. Hepatic blood supply may be reduced with starvation. BSP retention is increased, and serum bilirubin rises; unconjugated bilirubin more than doubles within 48 hours.3 Slight increases in the serum activities of aspartate and alanine aminotransferase and of lactate dehydrogenase are observed within 2 weeks of the start of a fast, but return to baseline within 4 to 6 weeks.34 Enzyme changes may be linked more to focal necrosis of the liver than to general circulatory impairment. In spite of the catabolism of tissue induced by starvation, the serum protein concentration is little affected initially; ultimately, a reduction occurs. With the onset of starvation, aldosterone secretion increases, leading to increased urinary excretion and decreased plasma concentration of potassium. Magnesium, calcium, and phosphate are affected similarly, although the urinary excretion of phosphate gradually declines. Although the plasma urea concentration is not significantly affected by 10 days of starvation, the absolute urinary excretion of urea, total nitrogen, and creatinine is increased over the first few days of starvation.15 Early in refeeding, sodium retention occurs as a result of decreased sodium and chloride excretion in the urine.77 The reduction in potassium excretion takes longer. These events are associated with an even greater secretion of aldosterone than occurs during the period of fasting. Abnormal concentrations of most constituents rapidly revert to normal with refeeding. Nitrogen balance soon becomes positive, especially if the nonprotein calories are derived mainly from carbohydrate.
Preanalytical Variables and Biological Variation
Preanalytical Variables
Controllable Variables
Physiologic Variables
Posture
Constituent
Average Increase, %
Alanine aminotransferase
7
Albumin
9
Alkaline phosphatase
7
Amylase
6
Aspartate aminotransferase
5
Calcium
3
Cholesterol
7
Immunoglobulin (Ig)A
7
IgG
7
IgM
5
Thyroxine
11
Triglycerides
6
Prolonged Bed Rest
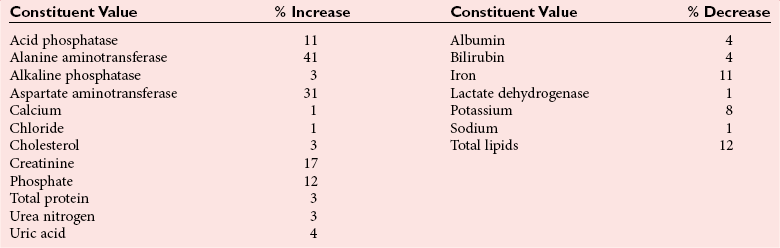
Exercise
Physical Training
Circadian Variation
Travel
Diet
Food Ingestion
Constituent
Before Meal
2 h After† Meal
Alanine aminotransferase, U/L
31
33
Albumin, g/dL
4.5
4.6
Alkaline phosphatase, U/L
46
46
Aspartate aminotransferase, U/L
22
28
Bilirubin, total, mg/dL
0.7
0.8
Calcium, mg/dL
9.9
10.0
Cholesterol, mg/dL
220.0
220.0
Glucose, mg/dL
71
82*
Lactate dehydrogenase, U/L
198
198
Phosphate, mg/dL
3.1
3.6*
Potassium, mmol/L
3.8
4.0*
Sodium, mmol/L
140
141
Protein, total, g/dL
7.8
7.9
Urea nitrogen, mg/dL
16
16
Uric acid, mg/dL
6.0
6.2
Ingestion of Specific Foods and Beverages
Vegetarianism
Constituent
Vegetarians
Nonvegetarians
S-Albumin, g/dL
4.2
4.3
P-Calcium, mg/dL
9.4
9.7
P-Cholesterol, mg/dL
213
252
P-HDL cholesterol, mg/dL
66
66
B-Glucose, mg/dL
90
101
B-Hemoglobin, g/dL
13.9
14.3
P-Triglycerides, mg/dL
106
124
B-Urea nitrogen, mg/dL
14
16
P-Uric acid, mg/dL
5.3
5.8
Malnutrition
Long-Term Fasting and Starvation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Preanalytical Variables and Biological Variation
