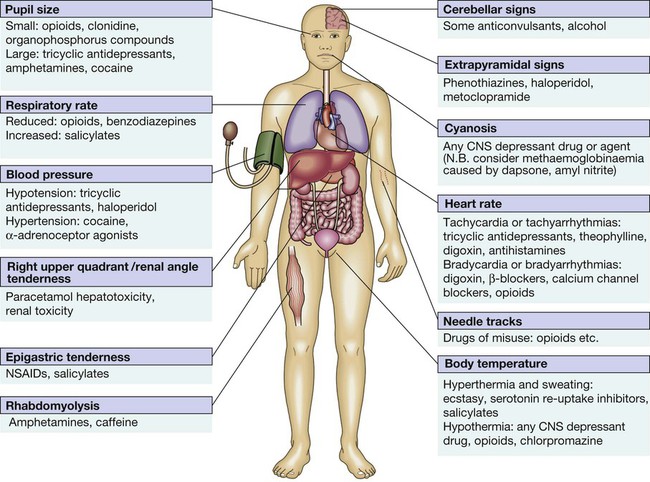
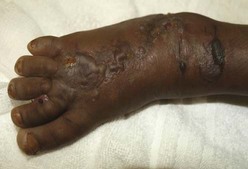
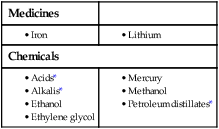
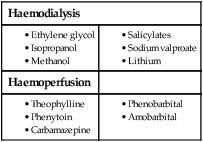
9 • What toxin(s) have been taken and how much? • What time were they taken and by what route? • Has alcohol or any drug of misuse been taken as well? • Obtain details from witnesses of the circumstances of the overdose (e.g. family, friends ambulance personnel) • Ask the general practitioner for background and details of prescribed medication • Assess suicide risk (full psychiatric evaluation when patient has physically recovered) • Assess capacity to make decisions about accepting or refusing treatment • Establish past medical history, drug history and allergies, social and family history • When was the patient exposed to a bite/sting? • Was the organism causing it seen and what did it look like (size, colour)? • What were the circumstances (on land, in water etc.)? • Was there more than one bite/sting? • What first aid was used, when and for how long? • What symptoms has the patient had (local and systemic)? • Are there symptoms suggesting systemic envenoming (paralysis, myolysis, coagulopathy etc.)? • Past medical history and medications? Acute poisoning is common, accounting for about 1% of hospital admissions in the UK. Common or otherwise important substances involved are shown in Box 9.1. In developed countries, the most frequent cause is intentional drug overdose in the context of self-harm and usually involves prescribed or ‘over-the-counter’ medicines. Accidental poisoning is also common, especially in children and the elderly (Box 9.2). Toxicity also may occur as a result of alcohol or recreational substance use, or following occupational or environmental exposure. Poisoning is a major cause of death in young adults, but most deaths occur before patients reach medical attention, and mortality is much lower than 1% in those admitted to hospital. A general approach is shown on pages 206–207. • immediate assessment of vital signs • identifying the poison(s) involved and obtaining adequate information about them • identifying patients at risk of further attempts at self-harm and removing any remaining hazards. Those with possible external contamination with chemical or environmental toxins should undergo appropriate decontamination (Fig. 9.1). Critically ill patients must be resuscitated (p. 180). Substances that are unlikely to be toxic in humans should be identified so that inappropriate admission and intervention are avoided (Box 9.3). History and examination are described on page 206. Occasionally, patients may be unaware or confused about what they have taken, or may exaggerate (or less commonly underestimate) the size of the overdose, but rarely mislead medical staff deliberately. In regions of the world where self-poisoning is illegal, patients may be reticent about giving a history. Toxic causes of abnormal physical signs are shown on page 207. The patient may have a cluster of clinical features (‘toxidrome’) suggestive of poisoning with a particular drug type, e.g. anticholinergic, serotoninergic (see Box 9.11, p. 213), stimulant, sedative, opioid (see Box 9.12, p. 217) or cholinergic (see Box 9.14, p. 221) feature clusters. Poisoning is a common cause of coma, especially in younger people, but it is important to exclude other potential causes (p. 1159), unless the aetiology is certain. Urea, electrolytes and creatinine should be measured in all patients with suspected systemic poisoning. Arterial blood gases should be checked in those with significant respiratory or circulatory compromise, or when poisoning with substances likely to affect acid–base status is suspected (Box 9.4). Calculation of anion and osmolar gaps may help to inform diagnosis and management (Box 9.5). For a limited number of specific substances, management may be facilitated by measurement of the amount of toxin in the blood (Box 9.6). Qualitative urine screens for potential toxins, including near-patient testing kits, have a limited clinical role. All patients presenting with deliberate drug overdose should undergo psychiatric evaluation by a health professional with appropriate training prior to discharge (p. 238). This should take place once the patient has recovered from any features of poisoning, unless there is an urgent issue, such as uncertainty about their capacity to decline medical treatment. Patients presenting with eye/skin contamination should undergo local decontamination procedures (see Fig. 9.1). Patients who have ingested potentially life-threatening quantities of toxins may be considered for gastrointestinal decontamination if poisoning has been recent (Box 9.7). Induction of emesis using ipecacuanha is no longer recommended. Given orally as slurry, activated charcoal absorbs toxins in the bowel as a result of its large surface area. If given sufficiently early, it can prevent absorption of an important proportion of the ingested dose of toxin. Efficacy decreases with time and current guidelines do not advocate use more than 1 hour after overdose in most circumstances (see Box 9.7). However, use after a longer interval may be reasonable when a delayed-release preparation has been taken or when gastric emptying may be delayed. Some toxins do not bind to activated charcoal (Box 9.8) so it will not affect their absorption. In patients with impaired swallowing or a reduced level of consciousness, activated charcoal, even via a nasogastric tube, carries a risk of aspiration pneumonitis, which can be reduced (but not eliminated) by protecting the airway with a cuffed endotracheal tube. Multiple doses of oral activated charcoal (50 g 6 times daily in an adult) may enhance the elimination of some drugs at any time after poisoning and are recommended for serious poisoning with some substances (see Box 9.7). This interrupts enterohepatic circulation or reduces the concentration of free drug in the gut lumen, to the extent that drug diffuses from the blood back into the bowel to be absorbed on to the charcoal: so-called ‘gastrointestinal dialysis’. A laxative is generally given with the charcoal to reduce the risk of constipation or intestinal obstruction by charcoal ‘briquette’ formation in the gut lumen. Gastric aspiration and/or lavage is very infrequently indicated in acute poisoning, as it is no more effective than activated charcoal and complications are common, especially aspiration. Use may be justified for life-threatening overdoses of some substances that are not absorbed by activated charcoal (see Box 9.8). Urinary alkalinisation is currently recommended for patients with clinically significant salicylate poisoning when the criteria for haemodialysis are not met (see below). It is also sometimes used for poisoning with methotrexate. Complications include alkalaemia, hypokalaemia and occasionally alkalotic tetany (p. 447). Hypocalcaemia may occur but is rare. These techniques can enhance the elimination of poisons that have a small volume of distribution and a long half-life after overdose, and are appropriate when poisoning is sufficiently severe to justify invasive elimination methods. The toxin must be small enough to cross the dialysis membrane (haemodialysis) or must bind to activated charcoal (haemoperfusion) (Box 9.9). Haemodialysis may also correct acid–base and metabolic disturbances associated with poisoning (p. 209). For most poisons, antidotes and methods to accelerate elimination are inappropriate, unavailable or incompletely effective. Outcome is dependent on appropriate nursing and supportive care, and on treatment of complications (Box 9.10). Patients should be monitored carefully until the effects of any toxins have dissipated. Management is summarised in Figure 9.2. Activated charcoal may be used in patients presenting within 1 hour. Antidotes for paracetamol act by replenishing hepatic glutathione and should be administered to all patients with paracetamol concentrations above the ‘treatment line’ provided on paracetamol poisoning nomograms. Acetylcysteine given intravenously (or orally in some countries) is highly efficacious if administered within 8 hours of the overdose. However, since efficacy declines thereafter, administration should not be delayed in patients presenting after 8 hours to await a paracetamol blood concentration result. The antidote can be stopped if the paracetamol concentration is shown to be below the nomogram treatment line. An alternative antidote is methionine 2.5 g orally (adult dose) every 4 hours to a total of 4 doses, but this is less effective, especially after delayed presentation. If a patient presents more than 15 hours after ingestion, liver function tests, prothrombin time (or international normalised ratio – INR), renal function tests and a venous bicarbonate should be measured, the antidote started, and a poisons information centre or local liver unit contacted for advice if results are abnormal. An arterial blood gas sample should be taken in patients with severe liver function abnormalities; metabolic acidosis indicates severe poisoning. Liver transplantation should be considered in individuals who develop life-threatening liver failure due to paracetamol poisoning (p. 932). Anticholinergic effects are common (Box 9.11). Life-threatening complications are frequent, including convulsions, coma, arrhythmias (ventricular tachycardia, ventricular fibrillation and, less commonly, heart block) and hypotension, which results from inappropriate vasodilatation or impaired myocardial contractility. Serious complications appear to be more common with dosulepin and amitriptyline. Activated charcoal should be administered if the patient presents within 1 hour. All patients with possible TCA overdose should have a 12-lead ECG and ongoing cardiac monitoring for at least 6 hours. Prolongation of the QRS interval (especially if > 0.16 s) indicates severe sodium channel blockade and is associated with an increased risk of arrhythmia (Fig. 9.3). QT interval prolongation may also occur. Arterial blood gases should be measured in suspected severe poisoning. In patients with arrhythmias, significant QRS or QT prolongation or acidosis, intravenous sodium bicarbonate (50 mL of 8.4% solution) should be administered and repeated to correct pH. The correction of the acidosis and the sodium loading that result is often associated with rapid improvement in ECG features and arrhythmias. Hypoxia and electrolyte abnormalities should also be corrected. Anti-arrhythmic drugs should only be given on specialist advice. Prolonged convulsions should be treated with intravenous benzodiazepines (see Box 9.10). There is anecdotal evidence of benefit from lipid emulsion therapy in severe intractable poisoning. Overdose of SSRIs may produce nausea and vomiting, tremor, insomnia and sinus tachycardia. Agitation, drowsiness and convulsions occur infrequently and may be delayed for several hours after ingestion. Occasionally, features of serotonin syndrome may develop (see Box 9.11), especially if SSRIs are taken in combination or with other serotonergic agents. Cardiac arrhythmias occur infrequently and most patients require supportive care only. The toxic effects of SNRIs are similar but tachycardia, hypertension or hypotension and ECG changes (QRS and QT prolongation) may be more prominent and hypoglycaemia can also occur. The major features of toxicity are bradycardia and hypotension. Heart block, pulmonary oedema and cardiogenic shock occur in severe poisoning. Beta-blockers with sodium channel-blocking effects may cause seizures, confusion and coma, while sotalol may be associated with repolarisation abnormalities (including QTc prolongation) and torsades de pointes (p. 570).
Poisoning
Comprehensive evaluation of the poisoned patient
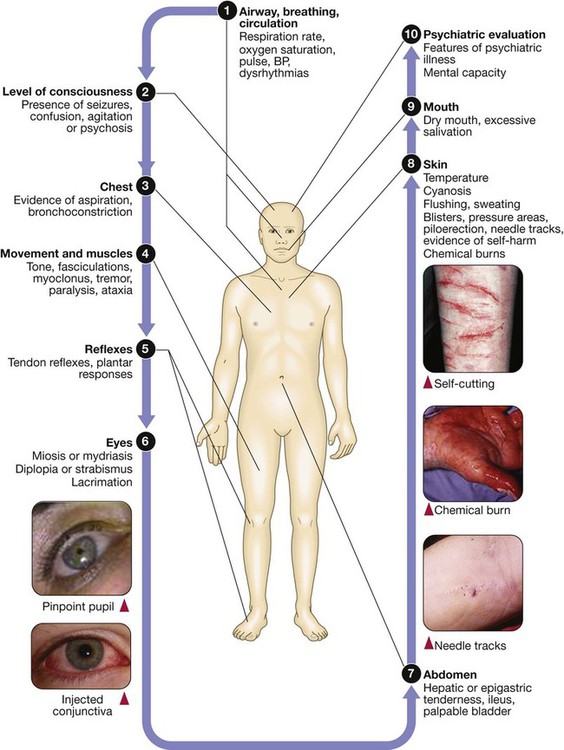
Taking a history in poisoning
Evaluation of the envenomed patient
Taking a history in envenoming
General approach to the poisoned patient
Triage and resuscitation
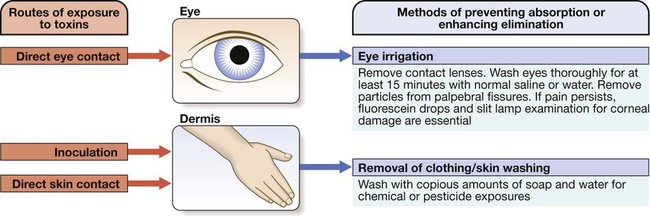
Clinical assessment and investigations
Psychiatric assessment
General management
Gastrointestinal decontamination
Activated charcoal
Gastric aspiration and lavage
Urinary alkalinisation
Haemodialysis and haemoperfusion
Supportive care
Poisoning by specific pharmaceutical agents
Analgesics
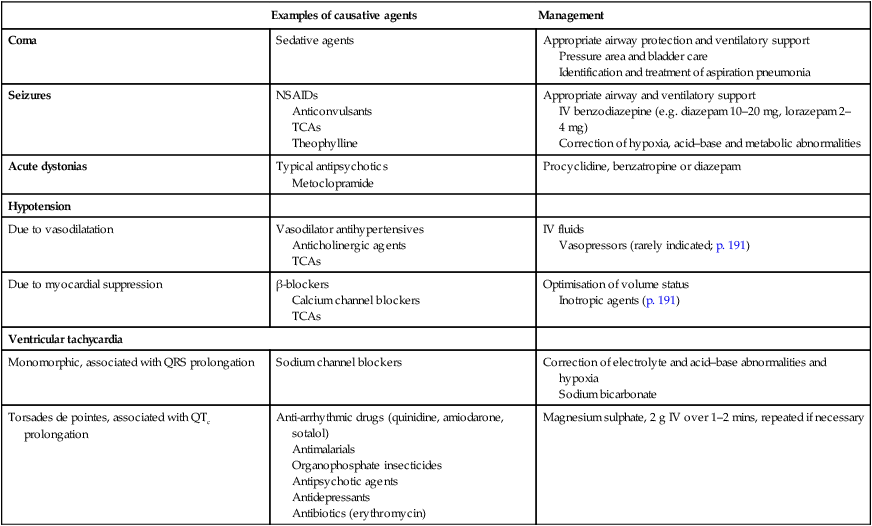
Paracetamol
Management
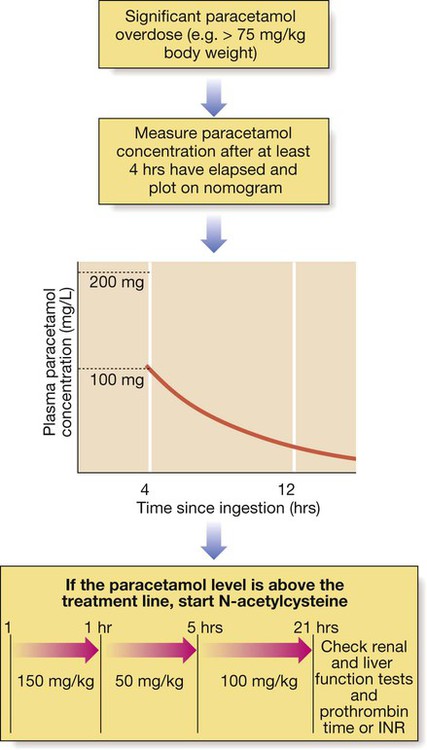
Antidepressants
Tricyclic antidepressants
Clinical features
Management
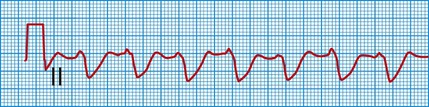
This rhythm strip shows a broad QRS complex due to impaired conduction.
Selective serotonin and noradrenaline re-uptake inhibitors
Clinical features and management
Lithium
Cardiovascular medications
Beta-adrenoceptor blockers
Clinical features
Poisoning