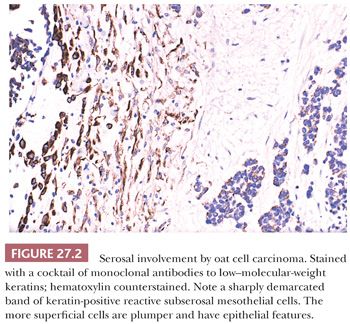
It is important to emphasize that these subserosal cytokeratin-expressing reactive cells, if not correctly identified, may be a source of diagnostic error, particularly in the distinction between desmoplastic malignant mesothelioma and fibrous pleurisy.
PLEURITIS
Acute pleuritis is typically either suppurative or fibrinous. Suppurative disease is typically infectious in nature, occurring with extension of a pulmonary bacterial infection. Abundant acute inflammation is present, extending throughout the pleural and subpleural tissues. Nonbacterial causes of acute pleuritis are associated with more fibrin and granulation tissue (fibrinous pleuritis) although acute inflammation is often still present. Within the edematous and fibrinous material, a proliferation of fibroblasts or submesothelial connective tissue and/or vascular tissue is often seen that may raise concern for malignancy. Although there may be some cytologic atypia with areas of increased cellularity with benign and reactive disease, the atypia usually resolves as one moves away from the pleural space toward the underlying lung or soft tissue (zonation).
Chronic pleuritis is typically only sampled if there is some degree of fibrosis (fibrosing pleuritis). The disease is often associated with chronic inflammation (lymphocytes and plasma cells) but it can be virtually acellular, with dense, parallel sheets of fibrosis (thus, at one end of the spectrum, it is histologically identical to an asbestos-related pleural plaque (see following discussion). Occasionally, the fibrosis can extend into the subpleural adipose tissue and one should be careful not to interpret this as desmoplastic mesothelioma (see following texts) (5). Adjacent to the pleural space, mesothelial hyperplasia may be present. Here, a vascular proliferation can also be seen with the reactive vessels coursing parallel to one another and perpendicular to the pleural space. Chronic pleuritis can be associated with collagen vascular disease or chronic irritation by another cause. Rarely, familial cases have been reported (6).
Granulomatous pleuritis refers simply to pleuritis associated with necrotizing or nonnecrotizing granulomatous inflammation. Foreign body type granulomas, replete with polarizable foreign bodies, are typically associated with talc. Infectious causes include tuberculosis, fungal disease, and other infectious agents. Rarely, the granulomas may be associated with rheumatic or autoimmune disease. Other variants of pleuritis include eosinophilic pleuritis (often associated with a history of pneumothorax) (7) and xanthomatous pleuritis (8).
NONNEOPLASTIC MESOTHELIAL PROLIFERATIONS
Unambiguous distinction between malignant epithelioid mesothelioma and adenocarcinoma is now relatively easy as a result of recent advances in immunohistochemistry. However, the distinction between mesothelial hyperplasia and mesothelioma continues to be a difficult diagnostic challenge for which immunohistochemistry is still of limited value (9).
Epithelioid Proliferations
Any chronic irritation may cause hyperplasia of the epithelioid mesothelial cells of the serous mesothelial surface as well as the submesothelial cytokeratin-expressing spindle cells. Because it may be very difficult to distinguish the epithelioid component of such hyperplasias from incipient, well-differentiated epithelioid mesothelioma, it is very important to base the diagnosis in such cases on a careful histologic evaluation with consideration of the total clinical and radiologic picture (9,10).
True invasion of the underlying tissue, particularly adipose tissue and skeletal muscle of the parietal pleura, remains the most reliable criterion of malignancy in epithelioid mesothelial proliferations. Here, it is important not to confuse entrapped mesothelial cells with invasion. Entrapped mesothelial cells are usually close to the pleural surface and are well delimited from the underlying adipose tissue, which they do not invade. Reactive fibrosis and inflammatory infiltrates are often present around and near the entrapped mesothelial cells (Fig. 27.3). Clearly, generous and nonfragmented biopsy specimens are essential to avoid error.
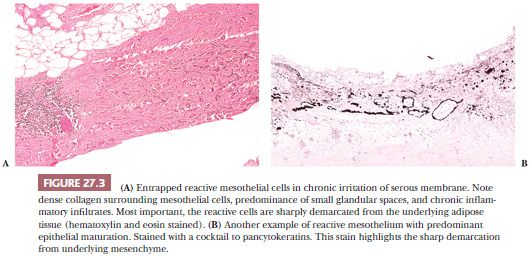
The appearance of the pleural surface, particularly at the time of thoracoscopy or thoracotomy, plays an important role in this differential diagnosis. The presence of confluent tumor nodules over large areas of the pleural surface favors malignancy. Absence of such nodules favors a reactive process. Additionally, malignant mesothelioma may be made up of monotonous tumor cells with little or moderate atypia, whereas reactive mesothelial cells may show similar atypical features. Thus, cytologic atypia, unless severe, may not be a reliable criterion for malignancy. Of note, the presence of tubular and papillary patterns of growth favors mesothelioma because these patterns are rarely seen in mesothelial hyperplasia.
Fibrous Proliferations
In the distinction between fibrous pleuritis and desmoplastic malignant mesothelioma, particular attention should be placed to the presence of zonation because it strongly favors a reactive process (9). The term zonation means a higher cellularity (and at times atypia) of spindle-shaped, cytokeratin-positive mesothelial cells toward the pleural surface, with less cellular and more collagenous layers beneath. Often, fibrinous deposits and capillaries growing perpendicular to the surface are noted in these reactive processes. However, it is important to emphasize that a reactive and fibrous pleuritis may be caused by the presence of an underlying malignancy, including mesothelioma. Ample and deep sampling, as well as partial decortication of the parietal pleural lesions, is thus very helpful. It cannot be overemphasized that a diagnosis of desmoplastic malignant mesothelioma must rest on the clear evidence of invasion.
ANCILLARY STUDIES FOR DISTINGUISHING HYPERPLASIA AND NEOPLASIA
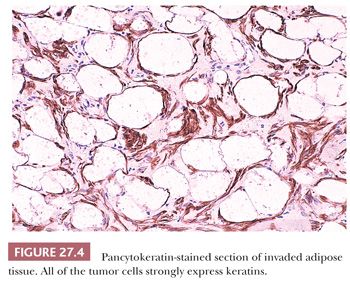
Immunohistochemistry, as will be discussed later in greater detail, helps to identify the mesothelial lineage of the proliferating cells but is of limited value to distinguish between mesothelial hyperplasia and mesothelioma. However, pancytokeratin stains may be useful to identify invasion (Fig. 27.4).
Although some have reported that p53 protein is frequently overexpressed by mesothelioma and not by reactive mesothelial proliferations (11–13), others have found otherwise (14–16). Moreover, studies using molecular biologic methods have revealed a low rate of p53 mutations in mesothelioma (16). Nonetheless, in an isolated case, strong nuclear immunostaining for p53 by the majority of the mesothelial cells supports mesothelioma over hyperplasia.
Some (17) have reported that GLUT-1, a member of the family of glucose transporter isoforms, may be a potential marker for distinguishing benign reactive mesothelium from malignant epithelial mesothelioma. They reported GLUT-1 expression in 100% of 40 mesotheliomas and in none of 40 cases of reactive mesothelium. Others (18,19) have not shown as robust results, and staining should be interpreted with caution.
Chiosea et al. (20) have reported that fluorescence in situ hybridization (FISH) analysis on paraffin-embedded tissue demonstrated the homozygous deletion of the 9p21 locus in 35 (67%) of 52 pleural mesotheliomas and in none of 40 cases of reactive pleural mesothelial proliferations. These results have been validated by others, and loss does seem to be specific for mesothelioma when compared to benign mesothelial proliferations (18). Immunohistochemical staining for loss of the p16 does not achieve the same specificity (20).
Other markers have also been suggested for distinguishing benign and malignant mesothelial proliferations, especially with cytology specimens that, by their nature, cannot demonstrate actual invasion. These include immunohistochemical staining for IMP3, desmin, XIAP, and EMA, among others (21).
Determination of DNA aneuploidy on serous effusions may serve as a marker for malignant cells. However, its usefulness to distinguish mesothelioma from carcinoma is controversial. El-Naggar et al. (22) compared the DNA flow cytometric characteristics of 23 epithelioid mesotheliomas and 41 pulmonary adenocarcinomas from paraffin-embedded blocks. They reported that 78% of the mesotheliomas were diploid versus a statistically significant 88% aneuploidy rate for the adenocarcinomas. Additionally, these authors found a significantly higher proliferative rate in adenocarcinoma than in mesothelioma. Similar findings were reported by Esteban and Sheibani (23). Thus, it would appear that the finding of a diploid tumor by flow cytometry favors a diagnosis of mesothelioma over adenocarcinoma. Pyrhönen et al. (24), by using fresh-frozen tumor samples, reported 16 (52%) of 31 mesotheliomas as diploid and found that the ploidy status was of no prognostic value in mesothelioma. Similar lack of prognostic value was reported by Dazzi et al. (25). Frierson et al. (26) studied effusion fluids and compared them with paraffin-embedded samples. They found a 53% rate of aneuploidy in mesothelioma and concluded that aneuploidy in an effusion specimen containing atypical mesothelial cells would strongly support a diagnosis of mesothelioma.
FIBROSIS AND PLEURAL PLAQUE
Chronic injury to the pleural surface may result in the formation of dense layers of scarlike tissue involving visceral as well as parietal pleura (see earlier discussion). Among causes of pleural fibrosis are asbestosis and other pneumoconiosis, inflammatory pleurisy including rheumatoid pleuritis, and, most commonly, bacterial pneumonias. Pleural fibrosis also may result from the intentional introduction of irritants (such as talcum powder) into the pleural cavity to promote therapeutic pleural fusion (pleurodesis).
A frequent area of diagnostic difficulty is the distinction between fibrous pleuritis and desmoplastic malignant mesothelioma, and this is now one of the most common requests to the United States–Canadian Mesothelioma Reference Panel (9). This difficult differential diagnosis will be discussed in some detail later.
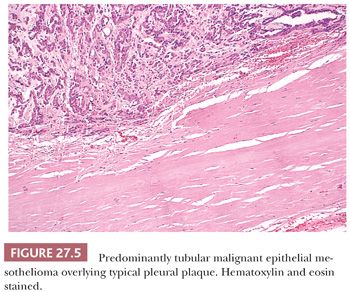
These forms of pleural fibrosis should not be confused with pleural plaques, which are, in the vast majority of cases, caused by asbestos exposure (27,28). Pleural plaques arise over the parietal pleura particularly at the lower chest and the diaphragmatic pleura. Usually, they develop at least two to three decades after asbestos exposure. Grossly, they consist of well-delimited, irregularly shaped, raised, grayish-white to ivory plaques ranging to several centimeters in size. They have a cartilaginous consistency and often calcify. Microscopically, they consist of dense strands of virtually acellular, intensely hyalinized collagen fibers that feature a reticulated, meshlike appearance, a pattern that has been referred to as “basket weave” (Fig. 27.5). Pleural plaques are clinically silent but serve as a reliable marker of asbestos exposure. It has been shown that pleural plaques contain asbestos fibers (29–31).
MESOTHELIAL TUMORS
ADENOMATOID TUMOR (BENIGN LOCALIZED EPITHELIAL MESOTHELIOMA)
Adenomatoid tumors of the pleura are exceedingly rare. The few that have been reported were found incidentally at lung resection for other conditions. Histologically, they were similar to the more common pelvic and peritoneal adenomatoid tumors (32–34).
MALIGNANT MESOTHELIOMA
Etiology, Incidence, and Pathogenesis
Since 1960, after pioneering studies on the incidence of mesothelioma in South African asbestos miners by Wagner et al. (35), the mesothelial carcinogenesis of inhaled asbestos fibers has been firmly established (36–42). Moreover, a correlation between the intensity and duration of exposure to asbestos fibers and the risk of developing mesothelioma is well documented (43,44). It also is evident, however, that some mesotheliomas, especially many of those occurring in young people, those in women, and those of the peritoneum, may not be related to asbestos exposure (45). The incidence of asbestos-related mesothelioma cases has ranged in different series from 10% to 99% (46). Although non–asbestos-related pleural malignant mesotheliomas undoubtedly exist, it remains difficult to determine their true incidence for two main reasons: (a) there is no defined threshold of exposure to asbestos fibers in relation to the development of mesothelioma (additionally, the issue of threshold is complicated by the possible effect of individual susceptibility [47]); and (b) there is a decades-long period of latency between the exposure to asbestos and development of the neoplasm (43,48). It was suggested (49) that even smaller exposures, such as that which may occur in the household, may be sufficient to induce mesothelioma. In a review of 668 cases of malignant mesothelioma, McDonald and McDonald (50) found that only 50% of the cases in men and 5% of the cases in women were associated with occupational exposure to asbestos. However, Vianna et al. (49) proposed that the wide variation in estimates of asbestos exposure in patients with malignant mesothelioma was a result of history taking.
Experimental and epidemiologic evidence suggests that other etiologic agents may be associated with mesothelioma as well (39,50–56). Such agents include radiation; minerals such as erionite, silica, and beryllium; and synthetic fibers, although evidence for the latter is far from conclusive (43). The finding of Simian virus 40–like DNA sequences (SV40) in some cases of human malignant mesothelioma suggested that the SV40 virus, which was a contaminant of some polio vaccines, may be implicated as a co-carcinogen or as directly causing mesothelioma (57,58). However, more recent studies report a low prevalence of SV40 in mesothelioma patients and suggest that earlier reports were a result of laboratory contamination (59). Of additional significance is the fact that several epidemiologic studies have failed to support an etiologic role of SV40 in mesothelioma (60–65). Finally, our understanding of genetic predisposition in the development of mesothelioma continues to evolve. Epidemiologic studies and reports of a number of kindreds have suggested that genetics plays some role in the development of some mesotheliomas (66–68). Recently, a familial cancer syndrome associated with germline mutations of BAP1 and the development of mesothelioma, uveal melanoma, and other cancers has been described (69–71).
Malignant mesothelioma is a rare tumor. McDonald and McDonald (50) estimated the combined United States–Canadian incidence to be 2.8 per million male and 0.7 per million female persons but then noted a steady increase in cases in men, which they attributed to occupational exposure to asbestos. A significant increase in the incidence of pleural mesothelioma among white men older than 55 years during the years of 1973 to 1980 was reported by Spirtas et al. (72) after a study of incidence rates based on data from population-based cancer registries in New York State (exclusive of New York City) and Los Angeles County (California) and the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute. In this study, even after histopathologic review, a notable upward trend remained. The tide has recently begun to change, however, and the incidence of pleural mesothelioma has started to decline in the United States (73).
The pathogenesis of malignant mesothelioma remains unclear. The presence of asbestos fibers in the vicinity of the serosal surfaces appears to be a crucial pathogenic factor. In both epidemiologic and experimental studies, differences in the tumorigenicity of asbestos fibers have been found, depending of the composition and physical characteristics of the fibers. Contributions of other factors (including heredity and exposure to other carcinogens such as tobacco smoke) to the pathogenesis of mesothelioma are not clear in most cases. Nonetheless, there is no compelling evidence that smoking increases the risk for the development of mesothelioma in asbestos workers (74).
Clinical Features
Malignant mesothelioma of the pleura is at least three times more common in men than in women (50,75). Most cases occur in patients between ages 50 and 70 years. Chest pain and shortness of breath are the most frequent initial symptoms; these are followed by weakness, fatigue, and weight loss. Clinical signs of pleural effusion are by far the most common finding at the initial physical examination and, in some cases, may precede the development of clinically detectable mesothelioma by several years (76). Chest roentgenograms or computerized tomography will reveal irregular pleural thickening that is most apparent after evacuation of the pleural fluid. Irregular thickening of the interlobar fissures is another characteristic radiographic feature of pleural mesothelioma. In patients who have had significant exposure to asbestos fibers, the presence of pleural plaque will frequently be discovered by these examinations. The effusions, which often are bloody, tend to recur rapidly after evacuation, but with progressive obliteration of the chest cavity by the growing tumor, they may subside. There may be involvement of pericardium and mediastinum as well as invasion of the soft tissues of the chest wall, particularly at biopsy sites or at the location of chest tubes after surgery. Metastases are uncommon in the early stages of the disease, but they may be seen in later stages and are a frequent finding at autopsy. Rarely, cases of metastatic mesothelioma with initial presentation as lymph node metastases have been reported, although many of these have been peritoneal mesotheliomas (77).
Historically, the average survival time from the onset of symptoms is approximately 15 months, but much longer survivals have been reported (78,79). Patients with pure epithelioid mesotheliomas tend to survive longer than those with sarcomatoid mesotheliomas (75,80). The treatment of mesothelioma is generally ineffective. Surgery is of limited benefit for pleural mesothelioma, although extrapleural pneumonectomy and extended pleurectomy and decortication are advocated by some. Radiation therapy and intracavitary instillation of radioactive substances have not been shown to be effective other than for palliation (38,81,82). However, combinations of surgical resection, radiation, and chemotherapy have been found to prolong life in selected cases (83). Overall, the literature regarding treatment for pleural mesothelioma is hampered by the fact that true randomized controlled studies have not been performed in spite of the fact that therapies are often extremely morbid. It can be said that healthier patients with lower stage tumors tend to survive longer and tend to undergo more aggressive therapies.
Pathologic Findings
Gross Findings. The gross appearance of mesothelioma depends on its stage at diagnosis. When diagnosed early, it appears as numerous small nodules or plaques extending over the visceral and parietal pleural surfaces. Later, confluence of the nodules results in a rindlike mass encasing and compressing the lungs. Usually, the tumor is thickest in the lower portions of the lung and over the diaphragm. At autopsy, invasion of the chest wall, lung, and mediastinum and distant metastases are common. The tumor may be firm, yellowish, and leathery, particularly in the desmoplastic variant, or it may be soft and gelatinous, especially in the poorly differentiated epithelioid types. In the latter, abundant foci of degeneration and necrosis are commonly seen at autopsy. The gross appearance of mesothelioma may be complicated by its intermingling with asbestos-related fibrous plaque.
Classification. Three major histologic types of diffuse malignant mesothelioma are described: epithelioid, sarcomatoid (fibrous), and biphasic (mixed); in each of these, cellular differentiation varies over a wide range. Diagnostic difficulties are presented by cases at all levels of differentiation. For example, well-differentiated epithelioid mesothelioma must be distinguished from reactive mesothelial hyperplasia, poorly differentiated epithelioid mesothelioma must be distinguished from metastatic undifferentiated carcinoma and other poorly differentiated malignancies, and epithelioid mesothelioma of intermediate degree of differentiation must be distinguished from pleural involvement by an adenocarcinoma of lung origin or from a distant metastasis from an adenocarcinoma arising elsewhere in the body. The distribution of the various histologic types varies from series to series, but usually epithelioid mesothelioma predominates. Because virtually all series are composed of consultation cases, they tend to underestimate the percentage of biphasic cases because these are easier to diagnose and less likely to require consultation.
Unusual Variants of Mesothelioma. Unusual variants include clear cell mesothelioma, a form of epithelioid mesothelioma that resembles metastatic renal cell carcinoma (84,85), and lymphohistiocytoid malignant mesothelioma, which mimics malignant lymphoma or other lymphoproliferative disorders (86,87). In these cases, immunohistochemistry is very helpful because the neoplastic cells express a mesothelial lineage immunophenotype. Another rare mesothelioma variant is the so-called deciduoid malignant mesothelioma, which is a large cell type of mesothelioma resembling exuberant ectopic decidual reaction. Although deciduoid mesothelioma is more commonly seen in the peritoneal cavity, cases have also been reported involving the pleura (88,89).
Localized malignant mesothelioma that is histologically and immunophenotypically indistinguishable from diffuse malignant mesothelioma has also been reported. These mesotheliomas exhibit a different biologic behavior than their diffuse counterparts, with many patients having a long survival after surgical resection (90,91).
Well-differentiated papillary mesothelioma (WDPM) is most commonly seen in the peritoneal cavity of women. It is characterized by papillary formations lined by bland epithelioid mesothelial cells with no or only superficial invasiveness. Like its peritoneal counterpart, WDPM of the pleura has an indolent clinical course (92,93).
Unusual Mimics of Mesothelioma. Infrequently, epithelioid vascular malignancies (hemangioendothelioma and angiosarcoma) involving the pleura may also enter the differential diagnosis with mesothelioma (see following discussion) (94–97). Thymomas arising from ectopic thymic tissues may rarely present as pleural-based tumors, occasionally with total encasement of the lung and clinically and macroscopically mimicking malignant mesothelioma. Histologically, they are indistinguishable from classical mediastinal thymomas (98,99). However, distinction from mesothelioma, particularly its lymphohistiocytoid variant, may be difficult on morphologic grounds alone, particularly with scant or fragmented biopsies. In these circumstances, immunohistochemistry can be very helpful because the epithelial cells express p63 and the lymphocytes show immunophenotypic features of T lymphocytes and express terminal deoxynucleotidyl transferase (TdT) (100,101). Synovial sarcomas, hematolymphoid malignancy, and other small blue cell tumors may also involve the pleura and can simulate mesothelioma clinically and histologically (see following discussion).
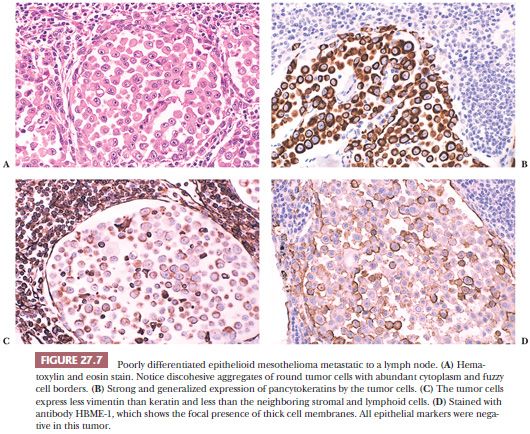
Histologic Features and Differential Diagnosis. The epithelioid type, if sufficiently differentiated, is characterized by predominantly papillary and tubular patterns of growth (Figs. 27.5 and 27.6). A variable proportion of solid growth, sometimes with a marked discohesiveness of the tumor cells, may be present in the less differentiated types and is the prevailing histologic pattern in poorly differentiated epithelioid mesothelioma (Fig. 27.7). The tumor cells are usually cuboidal, with a bulging, domelike apical portion, but may be flattened and rarely columnar. A common finding in well-differentiated epithelioid mesothelioma is the presence of single or multiple, sharply delimited, clear, round cytoplasmic vacuoles. These resemble those commonly seen in adenomatoid tumors and, under electron microscopy (EM), are identifiable as intracytoplasmic lumina. Because adenocarcinomas, epithelioid hemangioendotheliomas, and other neoplasms may contain intracytoplasmic lumina, this finding is of limited diagnostic value.
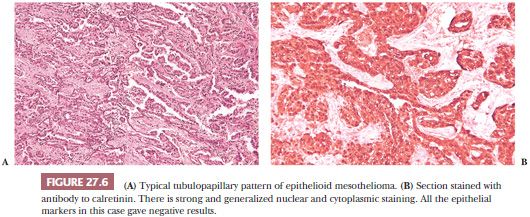
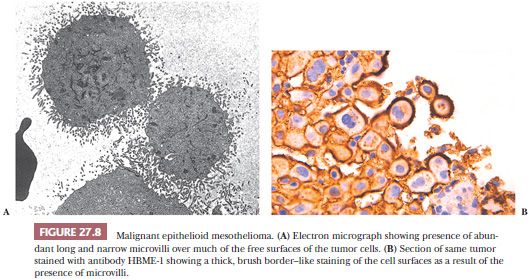
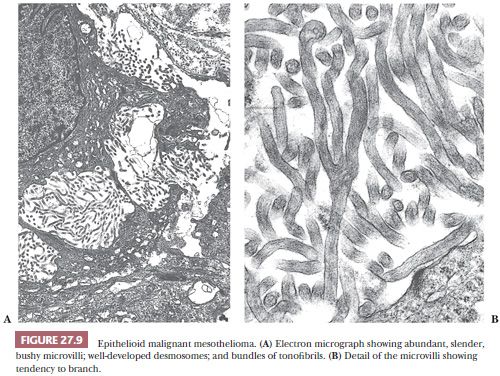
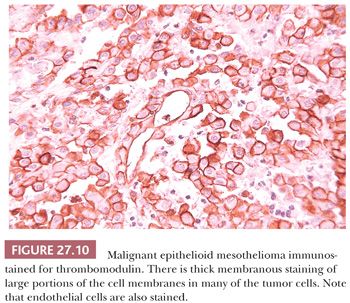
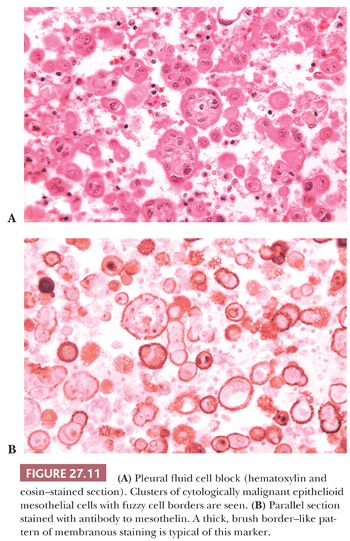
A brush border–like rim—corresponding to the abundant long microvilli seen by EM—may be seen on the free portions of the cell surfaces in thin sections of well to moderately differentiated epithelioid mesothelioma (Figs. 27.8 and 27.9). This is useful in distinguishing epithelioid mesothelioma from adenocarcinoma because the latter seldom has thick brush borders. Several immunostains to membrane-based marker molecules help to highlight this feature (Figs. 27.8, 27.10, and 27.11). Microcalcifications (psammoma bodies) may be seen, particularly in the tubulopapillary forms of epithelioid mesothelioma. They are of no diagnostic value.
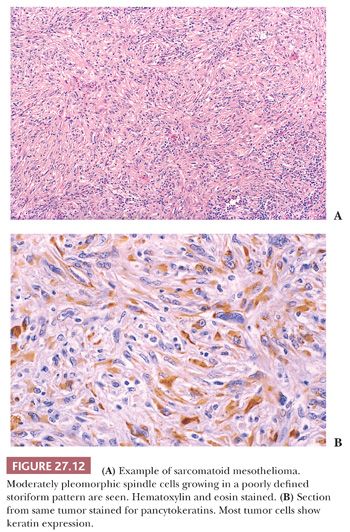
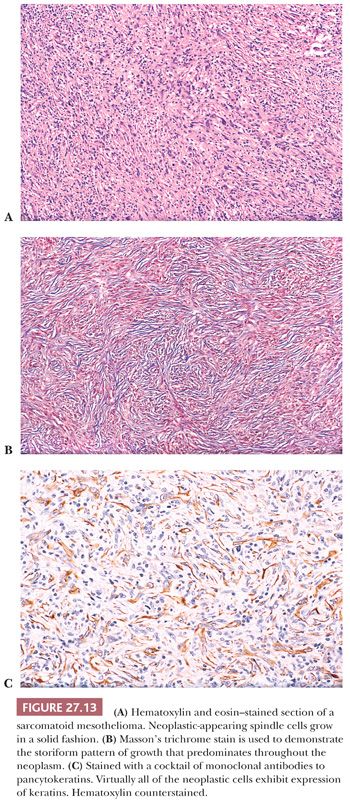
Diffuse sarcomatoid mesothelioma (sarcomatoid or fibrous mesothelioma) is made up predominantly of malignant-appearing spindle cells growing in short fascicles within a variable amount of fibrous stroma (Fig. 27.12). A storiform pattern of growth and focal hemangiopericytoma-like patterns are not uncommon (Fig. 27.13). Although not frequent, multinucleated atypical cells may be present and may give the neoplasm an appearance resembling malignant fibrous histiocytoma. However, sarcomatoid mesothelioma may also be well differentiated, with the neoplastic cells having a bland cytologic appearance resembling reactive fibroblasts. Considerable amounts of collagenous stroma may separate the neoplastic cells (Fig. 27.14). Such well-differentiated sarcomatoid mesotheliomas may resemble fibromatoses and are often designated as the desmoplastic variant of malignant sarcomatoid mesothelioma (102). They differ from the fibromatoses in that their neoplastic cells consistently express cytokeratins. However, their distinction from reactive pleural fibrosis is often difficult, as discussed earlier. Sarcomatoid mesothelioma also may imitate solitary fibrous tumor, schwannoma, and other sarcomas (103).
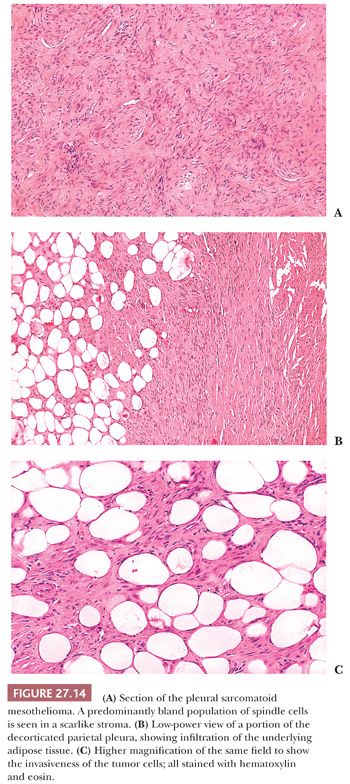
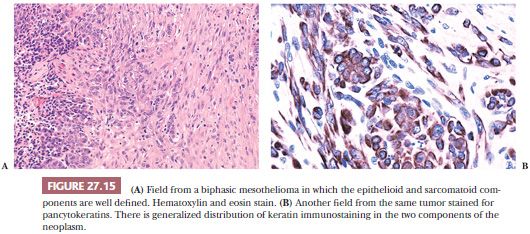
The mixed type, also called biphasic malignant mesothelioma, is the most easily diagnosed. As the name implies, it consists of a mixture of epithelioid and sarcomatoid cellular components (Fig. 27.15). For this reason, it has often been compared with synovial sarcoma, to which, however, it has only a superficial resemblance. Nonetheless, synovial sarcoma may present as a pleural-based lesion and mimic mesothelioma clinically and morphologically (104,105). The epithelial component of synovial sarcoma is often mucicarmine positive and expresses several epithelial markers by immunohistochemistry (106). Additionally, the translocation t(X;18) (SYT-SSX) characteristic of synovial sarcoma is not found in sarcomatoid mesothelioma (107). Sarcomatoid carcinoma of the lung with pleural involvement, a rare occurrence, may also simulate biphasic mesothelioma. Such cases can be identified by appropriate use of immunohistochemical procedures (see following texts) (108).
Clearly, malignant mesothelioma exhibits a wide range of differential diagnoses, from metastatic carcinoma of various origins to several types of sarcomas, as well as benign reactive processes. Although some cases of mesothelioma are sufficiently typical to permit a relatively firm diagnosis based on routine morphologic examination, in a large proportion of cases, its diagnosis is often imprecise and prone to be controversial. It had once been correctly stated that interobserver variability was greater for the diagnosis of malignant mesothelioma than for most other human neoplasms (109). Thus, numerous ancillary procedures, in particular histochemistry, EM, and immunohistochemistry, have been used in an attempt to improve the accuracy of mesothelioma diagnosis. Certainly, the widespread access to specific and sensitive immunohistochemical markers has done much to improve this problem over the years.
Adequacy of Biopsy Material. Decades ago, many believed that autopsy was the only way to ascertain a diagnosis of mesothelioma. Currently, with adequate biopsy, a firm diagnosis is nearly always possible. In many instances of differential diagnosis between malignant epithelioid mesothelioma versus adenocarcinoma, a needle biopsy, if representative of tumor tissue, may suffice. Indeed, with the help of immunohistochemistry, it is now possible, in many instances, to diagnose malignant epithelioid mesothelioma based solely on small samples. However, distinguishing between reactive hyperplasia and epithelioid mesothelioma or between fibrous pleuritis and desmoplastic malignant mesothelioma often requires more extensive sampling, preferably using thoracoscopy or thoracotomy. This is so because demonstration of invasiveness can be of foremost importance in the differential diagnosis in these cases.
Ancillary Diagnostic Procedures. Because no single test is currently capable of providing an unambiguous confirmation of the histologic diagnosis of mesothelioma, such diagnosis must be based on the accumulation of evidence. Nonetheless, recent advances in immunohistochemistry have markedly improved the accuracy of mesothelioma diagnosis to the point of rendering many older tests obsolete.
Histochemistry. Histochemical stains, such as mucicarmine, periodic acid-Schiff (PAS), and Alcian blue, once considered useful for the distinction between epithelioid mesothelioma and adenocarcinoma, have been largely superseded by the more specific and sensitive immunohistochemical stains.
Electron Microscopy. Before recent progress in immunohistochemistry, EM was often used to distinguish between epithelioid mesothelioma and adenocarcinoma. The ultrastructural hallmark of epithelioid mesotheliomas consists of the presence of long, thin, branching “bushy” microvilli, devoid of a glycocalyx coating, over much of their free cell surface (Figs. 27.8 and 27.9).
Warhol et al. (110) attempted to establish objective criteria for the ultrastructural diagnosis of mesothelioma and concluded that only the length-to-diameter ratio of the microvilli and the abundance of tonofilaments were of diagnostic value. Adenocarcinomas have fewer and shorter microvilli than do mesotheliomas. Additionally, microvilli in adenocarcinomas are usually present only on the apical portion of the cell surface, whereas in mesothelioma, they tend to involve all free cell surfaces (Fig. 27.8). Burns et al. (111) found a median length-to-diameter ratio of 11.9 in mesothelioma and 5.28 in adenocarcinoma. However, overlapping cases create a relatively wide “gray zone.” Dardick et al. (112) emphasized that poorly differentiated epithelioid mesothelioma and sarcomatoid mesothelioma may show no evidence at all of such long microvilli. Moreover, practically all mesotheliomas exhibiting long, sinuous microvilli also have a typical mesothelioma immunophenotype and often reveal thick brush border staining with membrane-based markers.
Distinction of sarcomatoid mesothelioma from true sarcomas is also simpler and more accurate by immunohistochemical means than by EM. Thus, EM is no longer needed for the diagnosis of mesothelioma, although it remains an essential tool for the identification and quantification of asbestos fibers in lung and pleural tissue (29,113).
Immunohistochemistry. Immunohistochemistry has evolved into the most important ancillary procedure in the distinction of epithelioid malignant mesothelioma from its mimics. However, there is yet no consensus about which is the ideal antibody panel, and there still remain some discrepancies on how best to interpret some of these immunostains.
Not too long ago, most relied chiefly on the use of antibodies binding to molecules expressed predominantly by carcinoma cells such as carcinoembryonic antigen (CEA), B72.3, CD15, and others. Thus, the diagnosis of mesothelioma was based on negative results, an unsatisfactory situation. This dramatically changed with the introduction of antibodies of relative specificity for mesothelial cells (Table 27.1). As a result, rarely are cases left undiagnosed when a well-chosen immunohistochemical panel is used. Because none of these new markers is by itself sufficiently specific or sensitive, they need to be used within a diagnostic panel. Clearly, as the number of available markers—particularly those of mesothelial lineage—continues to grow, pathologists need to periodically reevaluate their antibody panels to keep them reasonably sized with no loss of accuracy.
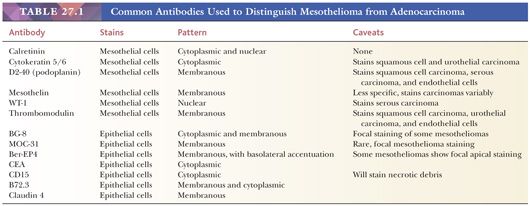
It is important to remember that specificity in immunohistochemistry is a relative concept because it depends in great part on the differential diagnosis in any particular case. For example, a partially mesothelial-restricted marker, such as Wilms tumor gene product (WT-1), has a very high specificity when the differential diagnosis is between mesothelioma and adenocarcinoma of the lung in a male patient. However, when ovarian carcinoma enters the differential diagnosis, this marker’s specificity dramatically decreases as it is expressed by a large proportion of serous carcinomas. Thus, awareness of each marker’s limitations is fundamental to select the antibody panel most suitable to the differential diagnosis at hand.
CYTOKERATINS (KERATINS). Keratins comprise a family of at least 20 individually gene-coded filamentous proteins that are the dominant cytoskeletal filament of epithelial cells. Which member(s) of the keratin family is expressed depends on the type of epithelial cell. Neoplasms derived from different epithelia tend to express the same combination of cytokeratins as their cell of origin. Thus, the cytokeratin immunophenotype of tumors is sometimes used as an indicator of cell lineage.
The cytoskeletal phenotype of mesotheliomas closely resembles that of reactive and resting mesothelium. When broad-spectrum antibodies (pancytokeratin antibodies)—recognizing epitopes shared by multiple members of the keratin family—are correctly used, almost all mesotheliomas and adenocarcinomas immunostain strongly and diffusely. Therefore, the use of pancytokeratin antibodies is not helpful for distinguishing mesotheliomas and carcinomas. However, pancytokeratin antibodies are valuable in the diagnosis of sarcomatoid mesothelioma and to identify invasiveness, particularly in the desmoplastic type.
Many monoclonal antibodies (MoAbs) recognizing epitopes restricted to certain members of the keratin family are currently available. Among these, one binds to an epitope shared by cytokeratins 5 and 6 and is of diagnostic value for epithelioid mesothelioma in paraffin-embedded tissues. Although MoAbs to cytokeratins 7 and 20 may help to pinpoint the site of origin of metastatic adenocarcinoma, they are of little value in the differential diagnosis between adenocarcinoma and epithelioid mesothelioma.
VIMENTIN. Vimentin, the chief intermediate filament of mesenchymal cells, is now known to be expressed by a large proportion of epithelium-derived neoplasms (114). Thus, earlier publications claiming usefulness of the demonstration of vimentin for the differential diagnosis between adenocarcinoma and mesothelioma are now less valid. In fact, some have found that a majority of epithelioid mesotheliomas do not express vimentin or do so minimally (115). However, vimentin, using clone V9 without antigen retrieval, is a useful universal control to detect overfixation and other types of antigen damage (116).
Mesothelial Markers (Positive Markers). This group consists of antibodies that bind to epitopes of molecules expressed mainly by normal mesothelial cells and their tumors. These antibodies should thus be regarded as markers of mesothelial lineage rather than as mesothelioma markers. As already stated, none of these is sufficiently specific or sensitive; thus, they need to be used within a diagnostic panel.
CALRETININ. Calretinin, a calcium-binding protein in the family of S-100 protein, was initially found to be present in central and peripheral neural tissues. Later, it was also found in adipocytes, renal tubular cells, Leydig and Sertoli cells, eccrine glands, and mesothelial cells (117,118). As is the case with S-100 protein, it is localized in both cytoplasm and nuclei (Figs. 27.6 and 27.16). Doglioni et al. (117) reported calretinin to be expressed by normal mesothelial cells and mesothelioma and rarely by adenocarcinoma. Several studies have confirmed a high sensitivity of this marker for epithelioid mesothelioma, while uncovering its expression by a small fraction of adenocarcinomas (119–121). However, although mesotheliomas usually immunostain strongly and diffusely, in the few positive adenocarcinomas, the staining tends to be weak and focal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


