The physical examination of patients with problem wounds should be focused on local and systemic signs that affect wound healing. For the wound itself, the location, size, depth, exposure of deep or vital structures, presence of necrotic material, presence of foreign bodies, or signs of any neoplastic processes should be carefully noted. The clinician often needs to consider bacterial colonization and acute/chronic infection as potential addressable causes. All open wounds will have bacterial colonization, but this by itself does not designate a wound as “infected.” The diagnosis of infection is contingent on the physical examination findings of local inflammation: erythema, pain, swelling, fluctuance, purulence, and loss of function. Physical examination should be the primary criterion for the diagnosis of local wound infection; surface swabs indicating the presence of pathogenic bacteria do not correlate with clinically significant infection.2 In addition to the wound itself, surrounding tissue should be examined for signs of injury (e.g., actinic changes), previous irradiation, arterial or venous insufficiency, lymphedema, loss of sensation, and dermal thinning (e.g., aging, steroid therapy). For all wounds on an arm or leg, a careful neurovascular examination for the entire limb is mandatory. In addition to the local examination, a focused systematic physical examination is mandatory in patients with problem wounds. Systemic signs of infection (e.g., fever, hypotension) are of particular importance. Obesity is a major risk factor that impairs wound healing. The general physical examination should focus on the systemic factors that affect wound healing as noted previously. Table 109-2 lists some of the local and systemic factors that impair wound healing; history and physical examination are the principal modalities for diagnosing these problems.
Laboratory examinations can be invaluable in the management of problem wounds. However, laboratory tests are often misused in wound patients, and a rational, evidence-based approach is necessary to efficiently utilize this expensive resource. Again, the local–systemic paradigm is useful in determining which laboratory examinations are warranted.
For local evaluation, wound swabs can be valuable for surveillance of the flora contaminating a wound but should not be used as a trigger to initiate therapy for wound infection. Wound biopsy and quantitative bacteriology have proved valuable in the management of burns and chronic wounds and can provide both topical and systemic antibiotic sensitivities.3,4 Bacterial loads in excess of 105/g tissue indicate contamination at a level that precludes skin graft take and jeopardizes wound closure of any kind. The use of quantitative cultures, however, is not justified for most acute or uncomplicated chronic wounds. In general, quantitative cultures are reserved for “high stakes” wounds, where a failure of closure on the initial attempt may leave an unreconstructable situation with grave consequences, such as amputation or death. Wound biopsies can be invaluable for diagnosing invasive burn wound infection and are preferred over quantitative culture for this purpose.5 The presence of bacteria in the deep dermis on biopsy is highly correlated with the risk of systemic sepsis in burn patients. Bone biopsy demonstrating bacteria within the bone is the gold standard test for making the diagnosis of osteomyelitis. Standard radiographs are most useful for diagnosing and delineating acute fractures and are much less useful in the setting of chronic, open wounds. Ultrasound, computed tomography (CT) scan, or magnetic resonance imaging (MRI) may be useful for delineating fluid collections, necrotic tissue, or inflammation in selected circumstances. In contrast, radionuclide bone scans have little role to play in patients with open wounds or fractures. In the face of an open wound or recent fracture, a “hot” bone scan (even a triple-phase bone scan) is not specific for osteomyelitis and has little value. Therefore, obtaining bone scans in patients with suspected sternal osteomyelitis after recent midline sternotomy, in pressure sore patients with exposed bone, or in other patients with open wounds overlying exposed bone is unwarranted. Under these circumstances, bone biopsy is preferred for making a diagnosis of osteomyelitis and for determining the responsible pathogen; MRI is preferred for delineating the extent of bony involvement. Computed tomography angiography (CTA), magnetic resonance angiography (MRA), or standard angiography may be indicated if vascular insufficiency is suspected or if a free-tissue transfer is planned. The choice of modality depends on both patient and health system-based factors.
CLASSIFICATION
Table 109-2 Local and Systemic Factors That Affect Wound Management

The use of systemic laboratory investigations should be limited to specific indications; “routine” blood work is not required for patients with acute or chronic wounds. White blood cell differential counts and blood cultures can confirm the diagnosis of systemic infection. Serum prealbumin may be valuable in determining nutritional status. A greatly elevated erythrocyte sedimentation rate and C-reactive protein can help confirm the diagnosis of osteomyelitis or be used to monitor treatment response. Other laboratory tests to confirm the diagnosis and severity of associated medical conditions may be justified for specific indications.
Treatment of the Problem Wound
Once a wound has been fully evaluated, the treatments should be designed to address any modifiable causes for the wound and then to achieve specific targeted goals. In order of greatest priority, these goals are: (a) prevention of complications resulting from the wound, (b) preserving or restoring critical functions, (c) achieving wound closure, and (d) restoration of aesthetics.
Preventive Treatment
The preventive measures that should be taken for patients with open wounds depend on the setting. For an acute laceration, tetanus prophylaxis should be considered. For patients with pressure sores, a strict adherence to pressure-relief protocols, optimization of wheelchair seating/bedding, optimization of social support and wound care, and an assessment of nutritional status take priority. In wounds caused by human or animal bites, prophylactic antibiotics are warranted. In addition, any associated medical conditions that are contributing to the wound must be aggressively optimized including glucose control, vascular disease, and contributing medications. It is the responsibility of the surgeon to ensure that a patient with a wound does not develop a complication from that wound and that the patient does not develop more wounds from the same mechanism. This is of particular importance in bedridden, obtunded, or paralyzed patients, in whom it should be possible to completely prevent pressure sores with proper nursing or family care.
Preservation of Function
Preserving joint motion must always be considered for patients with open wounds of the extremities. Aggressive physiotherapy to maintain or improve joint motion can be instituted in the presence of an open wound. Splinting should be used to minimize joint contractures and any plans for wound closure should include measures to maintain joint function. In the case of facial defects, especially if facial paralysis is present, oral competence and the maintenance of eye protection should weigh heavily into any reconstructive plan. Function takes precedence over form in the reconstructive algorithm.
Nonsurgical Therapy
After careful consideration of preventive measures and the preservation of critical function, a strategy for wound closure can be formulated. The basic tenet is: “débride dirty wounds, close clean wounds.” Therefore, the first step in wound closure is achieving control of the wound by eliminating necrotic debris and controlling any infection present. Nonsurgical therapies are used in conjunction with surgical therapy to achieve a clean wound. The mainstay of local, nonsurgical therapy is the use of wound dressings. It is beyond the scope of this chapter to review the wide range of options available to dress wounds. Recent articles contain a contemporary review of this topic.6–8 However, the basic principle is to employ débriding dressings for dirty wounds and occlusive dressings for clean wounds. The most commonly employed débriding dressing is the “wet-to-dry” dressing. Gauze made damp with normal saline, weak acetic acid, weak bleach, or various other solutions is applied to the wound. Over a period of hours, evaporation dries the dressing, which becomes slightly adherent to the wound surface. When the dressing is removed, necrotic debris and biofilm is removed with the dressing, but healthy tissue is left behind. Wet-to-dry dressings work through mechanical débridement, and the most important component of their use is how often they are changed. Wet-to-dry dressings must be changed a minimum of twice per day, although more frequent dressings may be necessary in certain wounds. These dressings should not be “soaked” off to reduce patient discomfort because this technique completely defeats their purpose. Enzymatic dressings have also been used to débride wounds, but their use can be limited by patient tolerance to the pain they cause. Débriding dressings are indicated for infected wounds and wounds containing necrotic debris.
If a wound is “clean,” meaning that it does not contain necrotic debris and has an acceptable bacterial load, a dressing that maintains a moist wound environment to encourage wound healing should be used.8 For many simple wounds, allowing a scab to provide the moist healing environment or the application of a nonadherent sterile dressing is all that is required. In the case of more complex wounds, occlusive dressings that maintain a moist environment to maximize wound healing are preferred. Options include hydrocolloid dressings, alginate dressings, and various hydrogels. Again, occlusive dressings must not be used on infected or dirty wounds. For many wounds, judicious application of hydrocolloid dressings may allow closure by secondary intention in a reasonable period of time.
Under some circumstances, it is appropriate to use antibiotic dressings. Multiple combinations of topical antibiotics are available over the counter and generally cover gram-positive bacteria. Mupirocin ointment has the added benefit for treating methicillin-resistant staph aureus (MRSA) colonization. Burn wounds are most commonly dressed with silver sulfadiazine or mafenide acetate due to their high antimicrobial activity. The low toxicity and excellent antibiotic properties of elemental silver have led to the development and widespread use of wound dressings containing nanocrystaline silver including both barrier dressings and in combination with alginates for daily wound care. In general, however, antibiotic dressings are not required for clean wounds, even if they are chronic.
Finally, it should be noted that we are entering a new era of nonsurgical wound management. A rapid advance in our understanding of wound healing has led to the development of growth factor therapy, cellular therapy, and new physical modalities for the treatment of chronic wounds.6 Although recombinant platelet-derived growth factor appears to be a useful adjunct to the dressing regime for pressure sores and diabetic foot ulcers,9,10 there is general agreement that exogenously administered growth factors currently play a limited role in the management of acute and chronic wounds.11 Similarly, laboratory data point to the potential for the use of stem cells as a modality to treat difficult wounds, but cellular therapy for clinical wounds is still largely investigational.6,12
There has also been an increase in tissue-engineered dressings and skin substitutes.13,14 For example, Integra (Integra Life Sciences Corp., Plainsboro, New Jersey), a bilaminar skin substitute composed of a collagen matrix base with a silicone rubber barrier layer has proven extremely useful in the management of complex wounds and allowing for granulation tissue in compromised wound beds in burns, trauma, and oncologic resections. The development of human, bovine, and ovine acellular dermal matrices are being used in wound care and structural reconstruction of the abdominal wall and breast.15 The development of new tissue-engineered adjuncts to wound healing remains a very active area of research in plastic surgery.
Negative-pressure wound therapy has become more widespread and can be efficacious and cost effective when applied appropriately for complex wounds.16–18 Multiple commercial products exist, but all are similar in the use of a sponge-like material connected to a suction device. There is additional variation in sponge material and inclusion of antimicrobial silver that can be used in specific clinical situations. Extensive experimental and clinical data confirm that the negative-pressure wound therapy promotes tissue perfusion, reduces edema, favorably alters wound fluid composition, and stimulates the formation of granulation tissue.18 Although these systems are easy to use, decrease the frequency of dressing changes, and can manage edema fluid, their use is contraindicated in acutely infected wounds and requires clinical judgment in balancing the tradeoff between negative-pressure therapy and other wound control methods.
Multiple other modalities, such as hyperbaric oxygen treatment, are in use and are being developed on an ongoing basis, underscoring the high prevalence, the significant cost, and the clinical challenge that are posed by complex wounds. Despite these ongoing developments, the basic algorithm for evaluation and nonsurgical management of wounds will not change.
Surgical Therapy
Wound Preparation. For problem wounds, surgical therapy is primarily aimed at wound preparation and wound reconstruction. For dirty wounds with necrotic debris, a judicious but thorough surgical débridement can convert a contaminated, chronic wound into a fresh surgical wound ready for immediate closure. Although débriding dressings can prepare wounds for closure under some circumstances, an operative débridement is preferred to a long course of débriding dressings for most problem wounds. Consequently, most complex wounds require an operative débridement prior to definitive reconstruction. In the case of chronic osteomyelitis, a formal resection of the sequestrum is required before formal wound closure. Prolonged treatment with intravenous or oral antibiotics cannot clear bacteria from a focus of dead bone; chronic osteomyelitis is a surgical disease cured with a saw, rongeur, bur, or bone curette. Therefore, the common practice of placing patients with chronic osteomyelitis on 6 weeks of antibiotic therapy is irrational unless performed in conjunction with a formal sequestrectomy.
ETIOLOGY
Table 109-3 The Reconstructive Ladder

Reconstructive Principles. As stated earlier, there is no “right” way to close any given wound. Plastic surgeons use a straightforward set of principles in delineating the optimum way to close a given wound for a given patient. The predominant principle is that the simplest method to close a wound is usually the best choice. This principle is embodied in the “reconstructive ladder,” which is a hierarchy of reconstructive options progressing from simple to complex (Table 109-3). Therefore, when engaging options for wound closure, plastic surgeons “climb” the reconstructive ladder, usually stopping on the lowest rung that will achieve a closed wound. However, other principles of reconstruction sometimes override a slavish adherence to the reconstructive ladder. The choice of a technique for wound closure should take into consideration the need for subsequent procedures and other factors that might mitigate skipping over simpler options for wound closure. An example would be an avulsion injury to the palm of the hand. Although it might be possible to close this wound with a skin graft, the need to restore flexor tendon function is preeminent in the hand, so that the use of a distant flap to provide a suitable bed for tendon grafting may be the preferred choice. In addition to the reconstructive ladder, other examples of guiding reconstructive principles are: function takes precedence over form, single-stage reconstructions are preferred over multistage approaches, and autologous tissue is preferred over alloplastic reconstructions. Other factors to be considered are the durability of the reconstruction over many years, the psychological impact on the patient, and data indicating that some options are sometimes preferred for specific reasons. For any reconstruction, the surgeon must carefully consider the potential secondary consequences of the reconstructive technique and morbidity at the donor site. The increased identification of alternative flap donor sites with less morbidity reconstruction has changed the reconstructive paradigm to sometimes use a “reconstructive elevator” to higher-level reconstructions to better address the secondary reconstructive outcomes. Thus, weighing this complex array of factors to arrive at the optimal reconstruction for a given patient is the true challenge in reconstructive surgery.
Reconstructive Techniques. Regardless of the reconstructive method chosen, plastic surgeons strive for technical virtuosity in the operating room. To maximize healing and minimize scar formation, atraumatic technique includes delicate tissue handling, the use of skin hooks and sharp rakes, bipolar electrocautery, sharp dissection, and loupe magnification. When reconstructing difficult wounds, the margin for error is minimal, and small errors in technical execution can result in failure. It is also critical that secondary plans for reconstruction are considered and preserved during an initial reconstructive attempt. The general surgical methods used by reconstructive surgeons are briefly considered in this chapter.
An important distinction is made between a graft and a flap. A graft consist of tissue that is transferred into a defect in a devascularized state and requires a vascularized environment and subsequent vascular ingrowth in order for incorporation. Skin, fat, muscle, tendon, fascia, cartilage, and bone can all be used as grafts; however, the volume of each that can be successfully incorporated depends on the surrounding wound environment and the physiologic principals of each tissue. In contrast, flaps consist of tissue that is transferred while remaining vascularized. Flaps are much less dependent on the local environment for survival given the inherent blood supply and can be used when immediate coverage is necessary or when the underlying defect will not support a graft. Free tissue transfer (or a “free flap”) is the transfer of tissue on a single vascular pedicle which is immediately anastomosed at the recipient site allowing for the transfer of vascularized tissue from a separate area of the body.
Primary Closure. If a laceration or other wound can be closed primarily, consideration is given to a meticulous, layered closure. Emphasis is placed on eversion of skin edges without strangling tissue. Nonabsorbable skin stitches that provoke minimal inflammatory response are preferred, but they must be removed promptly to minimize cross-hatching. Therefore, for most wounds, deep dermal, absorbable stitches are placed to allow early removal of skin stitches while still providing prolonged support to the repair; this may minimize the chances of a dehiscence or scar spread. It is preferable to place a closed suction drain to eliminate dead space rather than suturing fat or other easily devascularized tissue. If a technically perfect repair under physiologic tension cannot be achieved with primary closure, it is preferable to use a more complex surgical option.
Skin Grafting. Skin grafting was one of the foundations on which the specialty of plastic surgery was established. Skin grafts are classified as either split thickness, where epidermis and a portion of the dermis are harvested, or full thickness, where the epidermis and the entire dermis are harvested. Both full-thickness and split-thickness skin grafts can be used to resurface open wounds in cases in which primary closure is not possible. Skin graft take is contingent on the successful revascularization of the graft within a narrow time window (48 to 72 hours). Initially, grafts are nourished by a process of plasmatic imbibition, wherein serum from the wound bed diffuses into the adjacent graft. Revascularization occurs by the process of inosculation and angiogenesis. Vessels from the wound bed grow into the graft, forming functional circulatory connections with the vasculature of the graft. For plasmatic imbibition and inosculation to be successful, two criteria must be met. First, the wound bed must be appropriately vascularized. Therefore, skin grafts cannot be placed on poorly vascularized wound surfaces, including bone denuded of periosteum, tendon denuded of peritenon, or cartilage. Second, a bolster dressing or splint must be used to ensure absolute immobilization of the graft on the bed to prevent shearing of the nascent vascular connections during inosculation. If either of these criteria cannot be met, a more complex reconstructive option must be considered. Split-thickness graft donor sites heal due to epithelial cell growth from the base of remaining hair follicles and skin appendages. A full-thickness graft donor site must either be closed primarily or reconstructed with a different technique.
Random Skin Flaps. The use of tissue (usually skin) immediately adjacent to the defect as the tissue for reconstruction is referred to as a local flap reconstruction. Skin rearrangement can range in complexity from simple undermining to complex, geometric skin flaps. Which method is appropriate is dependent on multiple factors, including the etiology of the defect, the desired direction of the scar, the fragility or mobility of underlying structures, and the need to avoid distortion to adjacent free margins such as the lip or eyelid. Significant experience is required for the optimal utilization of skin rearrangement. When plastic surgeons think of “classical” skin flaps, they are referring to random skin flaps, without an axial blood supply. These skin flaps rely on a dermal plexus of vessels for their survival, and the perfusion of the distal end of the flap is inadequate to allow tissue survival if the flap design is inappropriate. It was previously thought that survival of the distal part of random skin flaps could be ensured by adhering to length-to-width ratios established for various areas of the body. It is now recognized that these ratios have no basis in circulatory physiology, and the surviving length of a random skin flap does not depend on flap width.19 In practice, most of the commonly employed skin flaps have been developed empirically. There are three basic types of random skin flaps: rotation, advancement, and transposition, depending on how adjacent skin is shifted into the defect. Each type of flap has specific design criteria and choice of flap design is based on wound location, patient anatomy, and surgeon preferences. Excellent reviews of the use of skin flaps in reconstructive surgery are available.20,21
As mentioned, the concern with flap viability limits the utility of random skin flaps. Several strategies have been developed to circumvent this problem. The first of these is the concept of surgical delay. Delay is defined as the partial interruption of blood flow to a defined piece of tissue allowing for preconditioning of the tissue prior to further division or transfer. At present, surgical delay is the only method available to augment the surviving length of random skin flaps (Fig. 109-1). Another strategy is the use of tissue expanders. Tissue expanders are implantable balloons that are inserted in a deflated state and are inflated with sterile saline via percutaneous injection into a self-sealing valve. Except for the scalp, where expanders are placed in the subgaleal plane, tissue expanders are usually placed subcutaneously. In fact, the insertion of the tissue expander into a subcutaneous pocket creates a surgical delay, and the slow expansion has been demonstrated to result in the formation of new skin. However, the most important strategy to circumvent the use of random skin flaps has been to develop axial pattern flaps that do not rely on a random blood supply.

Figure 109-1. Example of surgical delay. Because of associated medical problems, the patient was not a candidate for microvascular tissue transfer. A: Lower-third tibial defect secondary to an open ankle fracture. Note exposed bone, which precludes use of a skin graft. B: In the first stage, a bipedicle flap was created anterior to the defect. The deep perforators to the skin of the flap were divided by full undermining. The flap is perfused only from the proximal and distal ends. C: The incisions were repaired, and the wound was dressed. D: After 5 days, the bipedicle flap was again elevated and part of the distal pedicle was divided (arrow). The incisions were again closed, and the wound was dressed. This procedure was repeated 10 days after the initial operation to leave only a small skin bridge at the distal end of the flap. E: Fourteen days after the initial procedure, the remaining distal skin bridge was divided and the flap transferred. The donor site was skin grafted. This photograph depicts full survival of the flap and complete take of the skin graft.
Flaps with Axial Blood Supply. Regional flaps are defined as the transfer of tissue that is not immediately adjacent to the defect without the disruption of blood supply to the transferred tissue. For this approach to be practical, an axial blood supply to the transferred tissue must be present.
Axial Skin Flaps. The pioneering work of Bakamjian22 and McGregor and Jackson23 identified longitudinal blood vessels of large caliber traversing a defined region of skin. Their descriptions of the deltopectoral and groin flaps, respectively, opened a new era of reconstructive surgery. Surgeons were no longer constrained to the use of random skin flaps; any piece of tissue in the body with an axial blood supply could be transposed on a vascular pedicle with a high degree of assurance that the tissue would survive. Taylor and Palmer’s24 description of the angiosome concept solidified our ability to design flaps based on a sound knowledge of vascular anatomy. An angiosome is defined as a region of tissue supplied by an identifiable, and usually named, artery and its venae comitantes. Because of the ability of choke vessels crossing angiosome boundaries to enlarge, flaps can be designed to encompass an adjacent angiosome. In practice, there are relatively few axial skin flaps, most being better classified as fasciocutaneous flaps.
Fasciocutaneous Flaps. Cormack and Lamberty25 further expanded our understanding of the blood supply to the skin with their description of fasciocutaneous flaps. They pointed out that the dermal plexus derives its inflow from vertically oriented vessels arising at the level of the deep fascia. The vessels at the deep facial level have horizontal orientation relative to the skin’s surface and form a subfacial plexus. This subfacial plexus can form the basis for the design of fasciocutaneous flaps based on several defined anatomical patterns.25 Several fasciocutaneous flaps have enjoyed widespread use. The radial forearm flap has become a workhorse for hand reconstruction and as a free flap for head and neck reconstruction. The anterolateral thigh (ALT) flap has become a common flap for larger reconstructive needs and is based on septal or muscular perforators from the descending branch of the lateral femoral circumflex artery. The osteoseptocutaneous fibula free flap has been widely used for intraoral reconstruction during reconstruction of oromandibular defects and a primary site for vascularized bone flap harvest.
Muscle and Myocutaneous Flaps. Based on the knowledge that tissue with an axial blood supply can be reliably transferred, muscle and myocutaneous flaps came into widespread use in the 1980s, thanks in large part to the classification system by Mathes and Nahai.26 Whole muscles can be transferred as a pedicled or free flap if a dominant vascular pedicle is present. Segmental muscle transfers are sometimes possible on minor vascular pedicles. Functional muscle transfer can also occur when innervation is preserved or the motor nerve is coapted to a different motor nerve.
The rediscovery of the musculocutaneous perforator as a predominant vascular supply to the skin in many areas of the body has led to the wide use of musculocutaneous flaps. These flaps derive their inflow from a major muscular artery used to also bring skin along with the underlying muscle. Perforators emanating vertically from the muscle surface supply the skin overlying the muscle. Table 109-4 lists “workhorse” muscle and myocutaneous flaps. Of particular note is the transverse rectus abdominis myocutaneous (TRAM) flap, based on periumbilical myocutaneous perforators from the rectus abdominis muscle has been widely employed for breast reconstruction.27 An extension of this concept has led to the increasing use of “perforator” flaps. Perforator flaps are based on fasciocutaneous or myocutaneous perforating vessels. Dissection isolates the skin and subcutaneous tissue to be transferred on one or more perforating vessels; the underlying muscle is left intact, eliminating the functional disturbance that would result if a whole muscle was harvested. Because of their robust vascularity and ability to minimize donor deficit, perforator flaps are often preferred in reconstructions where muscle is not necessary and preservation of the underlying muscle decreases the morbidity at the donor site. An example is highlighted in the section on breast reconstruction where the use of the deep inferior epigastric perforator (DIEP) flap is based on the same donor site anatomy as a TRAM flap, but preserves the rectus abdominis muscle. In reconstructive surgery a large number of perforator flaps have been described and are now used as both pedicled and free flaps.28
CLASSIFICATION
Table 109-4 Workhorse Muscle, Myocutaneous, and Perforator Flaps

Microvascular Tissue Transfer. Historically, pedicled flaps were used to reconstruct distant defects. The distant transfer was accomplished in one of two ways. Flaps could be moved to the defect in a series of pedicled transfers (waltzing or tumbling flaps). Alternatively, flaps could be attached directly to the defect with a temporary pedicle to the donor site being maintained temporarily. At a second stage the flap was detached from the donor site, after it had developed sufficient blood supply from the recipient bed (e.g., cross leg flaps, pedicled groin flaps). Although the distant transfer of pedicled flaps still has definitive indications (e.g., the median forehead flap for nasal reconstruction), reconstruction using distant tissue is now most commonly performed via microvascular tissue transfer, or “free” flaps. First described clinically by Daniel and Taylor,29 free flaps have revolutionized reconstructive surgery. Any tissue with an axial blood supply with pedicle vessels 1 mm in diameter or larger can be reliably transferred microsurgically and improved techniques have opened the door to supermicrosurgery and the anastomosis of vessels between 0.3 and 0.8 mm in diameter.30 Circulation in the transferred tissue is established via microvascular anastomoses between the axial flap vessels and vessels in the recipient site. Microvascular anastomoses are performed with 8-0 or 9-0 suture with the aid of an operating microscope; success rates for free flap transfers exceed 95%.31 The greatest advantage of free flap reconstruction is that the surgeon is not restricted to the use of available, local tissues; any flap or composite block of tissue that has feeding vessels large enough for microvascular anastomoses can be transferred with this technique. By using composite tissue flaps, massive, complex tissue defects can be reconstructed replacing “like with like” (Fig. 109-2).32 The transfer of tissue to the site of reconstruction from another area of the body allows the use of tissue which has not been exposed to the damaging effects of radiation or scarring. Bowel, skin, bone, muscle, fascia, and composite tissue can be transferred microsurgically with new flaps continuing to be described clinically. Free flaps are the primary modality for reconstruction of major head and neck defects after composite resection and for the reconstruction of traumatic defects in the distal foreleg. As already mentioned, perforator flaps are increasingly used as microsurgical tissue transfers. The anterior lateral thigh (ALT) perforator flap has become a workhorse for reconstruction of a diverse array of complex defects, especially after extirpative surgery of the head and neck. The deep inferior epigastric artery perforator (DIEP) flap rivals the TRAM as the mainstay for autologous breast reconstruction.33
The future of reconstructive techniques continues to evolve. The use of prelaminated and prefabricated flaps can now be used to create complex reconstructions elsewhere on the body and then transfer that reconstruction en bloc to the recipient site.34 Tissue-engineered reconstructions are emerging with the modification of both biologic and nonbiologic tissues including the modification of stem cells, generation and cellularization of scaffolds, and three-dimensional (3D) printing for reconstructive purposes.35 In addition, vascularized composite allotransplantation (VCA) has now been successfully performed in multiple areas including both hand and facial transplantation.36 Continued work on immunosuppression modification should continue to shift the risk–benefit ratio toward standard reconstructive techniques.
RECONSTRUCTIVE AND AESTHETIC SURGERY OF THE BREAST
Plastic surgery of the female breast includes both reconstructive and aesthetic procedures. Breast reconstruction following mastectomy and reduction mammaplasty for macromastia (oversized breasts) are the most common examples of reconstructive breast surgery. By contrast, breast augmentation (enlargement) and mastopexy (breast lift) generally are performed for aesthetic reasons. Although some surgical approaches may be applicable to both categories of breast procedures, the relative benefits, risks, and costs of reconstructive versus aesthetic breast surgery may be quite different, particularly as viewed by patients, providers, and payers.
Before advising women on reconstructive or aesthetic breast procedures, the surgeon should carefully assess the patient’s current preferences, concerns, history, and physical findings. Initially, any history of breast disease (as well as familial history of breast pathologies) should be evaluated. On physical examination, careful linear measurements and preoperative photographs should be taken to document existing contour deformities, asymmetries, or other findings. A standard breast examination should be carried out to detect previously undiagnosed masses, nipple discharge, or lymphadenopathy. Finally, it is also advisable that women age 40 years or older undergo mammography unless this study has been performed in the previous 12 months.
Preoperative consultation should include a thorough discussion covering the relative benefits and risks of the various surgical options. The surgeon is well advised to provide comprehensive information in an understandable format. To enhance patient satisfaction with the eventual surgical result, providers should elicit patients’ preferences and expectations for surgical outcomes and tailor treatment options accordingly. Because of the prevalence of breast cancer in North American women, the impact of reconstructive or aesthetic procedures on breast cancer monitoring also should be discussed.
Reconstructive Breast Surgery
Postmastectomy Reconstruction
1 A variety of operative procedures have been described for breast reconstruction following mastectomy. These approaches can be categorized as implant-based, autogenous (natural) tissue, and “hybrid” procedures. For purposes of this discussion, the term hybrid is applied to procedures combining elements of both implant and autogenous tissue techniques. This overview describes the advantages and disadvantages of the most common techniques. Ultimately, procedure selection is based on a range of patient variables, including size and shape of the desired reconstructed breast; availability of local, regional, and distant donor tissues; coexisting medical problems; and, perhaps most important, patient preferences.
Implant-Based Techniques. As most commonly practiced, implant-based breast reconstruction is either performed as a staged tissue expander–implant reconstruction or as a “single stage” implant reconstruction. The tissue expander–implant approach usually requires two or more operative procedures to reconstruct the female breast. In the first stage, a temporary tissue expander is placed in a soft tissue pocket, usually located deep to the pectoralis major muscle. The tissue expander is a deflated silastic (silicone) envelope with an integrated or remote injection port through which saline solution can be percutaneously injected. Expanders can be completely covered with a combination of flaps including the pectoralis major (superior), serratus anterior (lateral), and rectus fascia (inferior). Alternatively, with the introduction of acellular dermal matrices (ADMs), expanders are now routinely covered with a pectoralis major muscle flap superiorly and ADM sling inferiorly. Following expander placement and meticulous closure of the overlying muscle and skin, saline is periodically injected beginning at 10 to 21 days. As the device enlarges, growth is induced in the overlying skin, recreating soft tissue coverage for the new breast.37
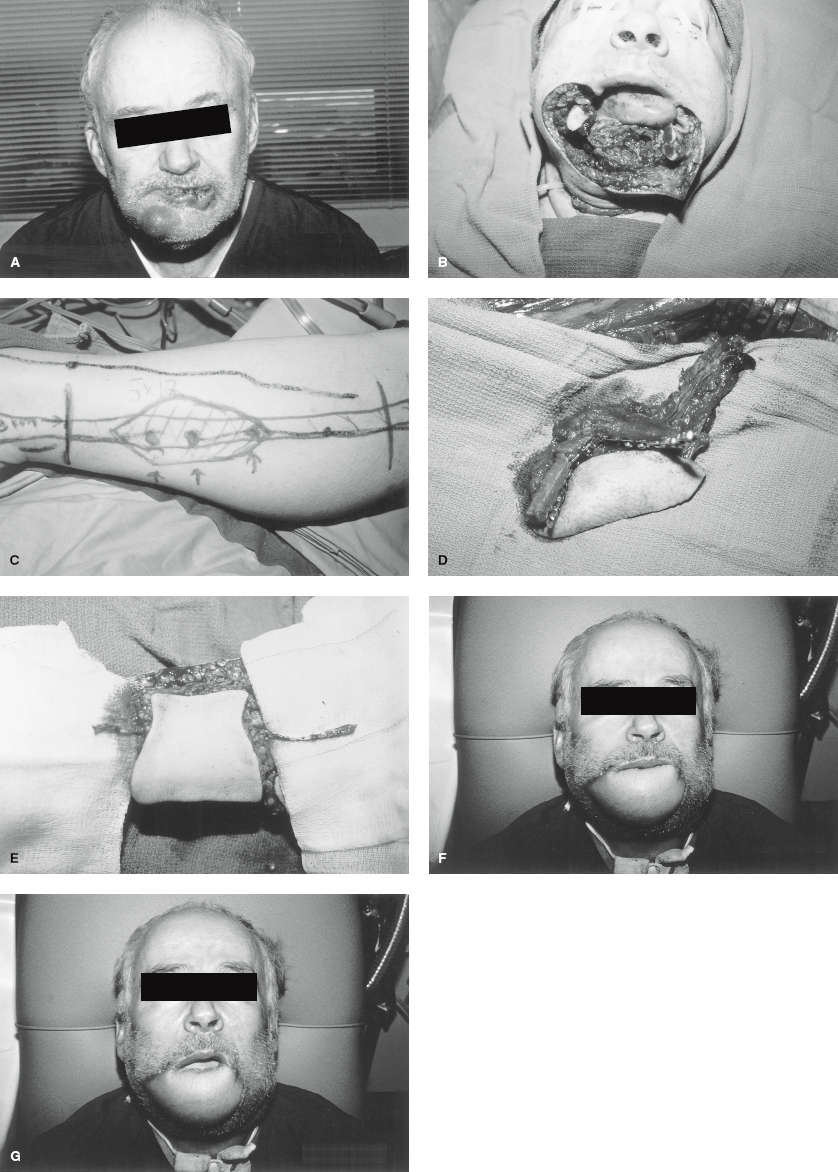
Figure 109-2. Double free-flap reconstruction of a massive, complex defect. A: Squamous cell carcinoma of lip, chin, mandible, and floor of mouth after resection. Massive tissue defect encompasses skin of the chin, entire lower lip, mandible from midbody to midbody, and floor of the mouth. B: Fibula free flap. Skin paddle is centered on fasciocutaneous perforators. C: Fibula free flap elevated but still in situ. Osteotomies have been performed to conform to the mandibular contour. The skin paddle will form the floor of the mouth. D: Radial forearm free-flap plan. Skin paddle will be harvested along with the palmaris longus tendon. The tendon will be attached to the modiolus on either side of the lower-lip defect, and the skin paddle will reconstruct the skin of the chin and lip. For the lip, the skin paddle will be draped over the tendon like a bed sheet on a clothesline. E: Radial forearm flap elevated but in situ. F: Postoperative result, mouth closed. G: Postoperative result, mouth open. (Reproduced with permission from Kuzon WM Jr, Jejurikar S, Wilkins EG, et al. Double free-flap reconstruction of massive defects involving the lip, chin, and mandible. Microsurgery 1998;18:372–378.)
Following completion of expansion, most surgeons delay the second stage of reconstruction for 1 to 4 months to allow for maximal skin growth. At the conclusion of this hiatus, the second procedure is performed, consisting of removal of the expander and placement of the reconstructive implant. Currently available breast implants include a range of options, including variations in shape (round vs. “anatomic” or teardrop configurations), fill material (silicone gel vs. saline), and surface configuration (smooth vs. textured envelopes). The relative advantages and disadvantages of these options are discussed in the Breast Augmentation section later in this chapter. As the most common option for postmastectomy reconstruction in the United States, the expander–implant approach offers several advantages. The lengths of the surgical procedures associated with this approach usually are relatively brief (often 1 hour or less) and are technically straightforward. Particularly when employed in bilateral reconstruction cases, resulting symmetry and aesthetic outcomes are relatively good (Fig. 109-3).
Patients considering implant reconstruction should also be mindful of the disadvantages of these procedures. The expander–implant approach usually requires at least two surgical procedures, multiple visits for expansion, and approximately 6 to 12 months to complete. For patients eager to return to a normal lifestyle following breast cancer treatment, this delay can be particularly frustrating. In addition, tissue expanders and reconstructive implants have been associated with a number of complications. Early in the postoperative period, these devices may be troubled by delays in wound healing, at times resulting in implant exposure and requiring explantation. Implant infections also may necessitate removal of the prosthetic device. Late complications include expander or implant leakage. Also, the development of excessive scar tissue surrounding the implant (termed a capsular contracture) can produce a hard, painful, or deformed breast requiring surgical revision.


Figure 109-3. Staged bilateral tissue-expander based breast reconstruction. A, B: Pre-operative apperance. C, D: After tissue expansion. E, F: Final result after expande/implant exchange and nipple-areolar complex tattoing.
The “single stage” approach to implant reconstruction bypasses the tissue expander stage, with immediate placement of a full-sized silicone or saline-filled implant.38 Similar to the tissue expander–implant reconstruction, patients will need revision procedures to achieve a complete reconstruction. The use of ADMs have made this option of reconstruction possible as larger volumes can be placed under an ADM; complete coverage with muscle limits the intraoperative volume placed in an expander at the initial reconstruction. Careful patient selection is necessary when considering this option and patients need to understand the reconstructed breast volume will be about the same size or smaller than their premastectomy volume. Patients who desire to be larger are better served with tissue expansion.
2 Autogenous Tissue Reconstruction. A variety of autogenous (natural) tissue options have been described for postmastectomy breast reconstruction. Currently, the most commonly performed of these procedures are based on the lower abdominal soft tissue. Originally popularized by Hartrampf et al.30 the TRAM (transverse rectus abdominis myocutaneous) flap was the earliest lower abdominal flap routinely used for breast reconstruction. The flap was commonly performed as a pedicle muscle flap, with transfer of the rectus muscle which is left partially attached to the costal margin, preserving the superior epigastric artery and vein as the flap’s blood supply (Fig. 109-4A and B). These tissues are tunneled subcutaneously into the mastectomy defect, where they are sculpted into the desired breast size and shape. Meanwhile, the abdominal donor site is closed by reapproximating the anterior rectus sheath and by advancing the remaining superior skin edge of the donor site as a modified abdominoplasty (Fig. 109-4C). As harvest of the pedicled TRAM flap requires use of an entire rectus muscle and a segment of the abdominal wall fascia, occurrences of postoperative abdominal hernias or (more commonly) abdominal wall laxity are a persistent problem, particularly in patients who undergo bilateral reconstruction using this technique.39
Microsurgical techniques for soft tissue transfer have allowed for modifications of the TRAM flap and have also encouraged the development of other alternative flap options. Other lower abdominal flap options requiring microsurgical free-tissue transfer include the free TRAM, muscle sparing TRAM, DIEP (deep inferior epigastric perforator) and SIEA (superficial inferior epigastric artery) flaps. Differences between these flap techniques are based on the amount of rectus muscle and fascia harvested with the flap, with the DIEP and SIEA flaps avoiding muscle or fascia harvest. Allen and Treece took the free TRAM flap a step further with their development of the DIEP flap: by meticulously dissecting vascular perforators from the deep inferior epigastric pedicle into the overlying abdominal fat, avoiding removal of any rectus abdominis muscle or fascia (Fig. 109-5A and B), thereby minimizing disruption of abdominal wall structures.40 This consequently decreases the occurrence of abdominal bulges or hernias.39,41,42 The flap is transferred to the chest where the flap vascular pedicle is anastomosed to recipient vessels in the chest with the aid of an operating microscope or high-power surgical loupes. The more common recipients utilized today are the internal mammary vessels (Fig. 109-5C), with the thoracodorsal vessels serving as a second option.
Abdominal soft tissue flap breast reconstruction offers several benefits. Because the flaps usually provide a generous amount of lower abdominal adipose tissue for breast bulk, implants are rarely needed with this approach (Figs. 109-6 and 109-7). The flaps can be inset and sculpted in a virtually infinite number of ways, giving the reconstructive surgeon considerable latitude in the creation of breast shapes and sizes. An additional advantage of abdominal-based flaps is their tendency to gain or lose volume in association with weight changes and body mass, thereby maintaining better symmetry than implant reconstructions over time. Finally, flap reconstructions avoid long-term problems encountered with implants such as implant rupture, which may necessitate additional surgical procedures. Likely based on these benefits, patients tend to be more satisfied with soft tissue flap breast reconstruction than implants in the long term.43 Despite these advantages, lower abdominal flaps also are associated with some disadvantages. Compared with implant approaches, flap reconstruction requires an abdominal scar, longer operations, hospitalizations, and recovery times. Furthermore, flap procedures can produce a range of complications, including partial and total flap loss as a result of vascular compromise of the transferred tissue.

Figure 109-5. Deep inferior epigastric artery perforator (DIEP) flap. A: abdominal donor site. B: Flap harvested without muscle or fascia. C: Microvascular anastomosis to internal mammary vessels.

Figure 109-6. DIEP breast reconstruction. A, B: s/p left mastectomy. C, D: After left DIEP flap reconstruction. E, F: Final result after tattooing of nipple-areolar complex.
The free flap concept also has been applied to other donor sites for breast reconstruction, most notably the thighs (transverse upper gracilis and profunda artery perforator flaps) and gluteal region (superior and inferior gluteal artery perforator flaps).44–47 Although perforator flaps appear to avoid the functional deficits associated with muscle flap harvest, they (like all free tissue transfers) entail longer, technically challenging operations requiring special facilities, equipment, and expertise. Also, because the blood supply for the entire flap depends on two or three microsurgical anastomoses (each usually involving vessels no more than 2 to 3 mm in diameter), there is the potential for complete flap loss in the event of anastomotic thrombosis. Flap loss rates are however relatively low at 1% to 2% in high-volume centers.
“Hybrid” Breast Reconstruction Techniques. As an additional alternative for breast reconstruction, flaps can be used in concert with saline or silicone-gel implants. Most commonly, the ipsilateral latissimus dorsi and a segment of overlying skin are harvested as a musculocutaneous pedicle flap and are tunneled anteriorly into the mastectomy defect. Although the latissimus dorsi and its associated skin island constitute an extremely reliable flap when used for wound coverage, this approach usually provides insufficient bulk for breast volume. Tissue expanders or implants are often used to address this volume deficiency. The combination of a latissimus dorsi flap and tissue expansion may be particularly appropriate for cases in which the remaining mastectomy skin is of insufficient quality or quantity to tolerate tissue expansion. Following transfer and expansion of a latissimus dorsi musculocutaneous flap, an appropriately sized breast implant usually can be safely placed in a secondary operation.
Nipple–Areolar Reconstruction. Reconstruction of the nipple–areolar complex (NAC) can be accomplished at the conclusion of breast mound reconstruction or at a later date. Following recreation of the mound, many surgeons prefer to allow several months for tissue healing and settling before proceeding with nipple–areolar reconstruction. To recreate the papule, common options usually rely on local skin flaps or a segment of redundant contralateral papule. For the areola, a full-thickness skin graft or tattooing of the surrounding skin can be used. In recent years 3D tattooing of the NAC has been introduced as an alternative to surgical reconstruction with good aesthetic results (Fig. 109-8).48
Breast Reduction (Reduction Mammoplasty)
Because reduction mammoplasty is intended to alleviate functional problems and symptoms of macromastia, this procedure is considered a reconstructive rather than an aesthetic operation. In general, appropriate candidates for reduction mammoplasty are women with macromastia and associated back, neck, or shoulder pain; limitations in daily work or recreational activities; or difficulties obtaining proper fit in bras or other clothing. Although reduction mammoplasty usually is a covered benefit by most health care payers, patients symptomatic and functional concerns must be carefully assessed and documented before proceeding with surgery. As noted earlier in this section, patients preferences and expectations regarding postoperative breast size, shape, and functional results also should be carefully evaluated.

Figure 109-7. DIEP breast reconstruction. A: Pre-operative appearance. B: Post-operative, showing abdominal donor site scars.

Figure 109-8. A: Standard nipple-areolar complex tattooing after nipple reconstruction. B: 3D tattooing of nipple-areolar complex without nipple reconstruction.
Although a variety of approaches have been described for breast reduction, common surgical options share a number of characteristics. Most techniques of breast reduction resect both breast parenchyma and redundant skin. Also, reduction procedures generally reposition the NAC to a more superior point on the breast mound. To maintain nipple viability and sensation, the NAC usually is mobilized as part of a pedicle of breast parenchyma or dermis. Following dissection of the nipple pedicle and reduction of the surrounding breast skin and parenchyma, the pedicle is transferred superiorly with its vascular and neural supplies intact, while the remaining breast is reapproximated around the nipple pedicle.
In categorizing reduction mammoplasty techniques, surgical options often are described in terms of the nipple pedicle design. For example, the most common approaches rely on an inferiorly or centrally based dermal–parenchymal pedicle to maintain vascular and nerve supplies to the NAC (Figs. 109-9A to C and 109-10A to C).49–51 In designing skin incisions, traditional methods of reduction often have incorporated a modification of a pattern originally described by Wise.52 While allowing considerable flexibility in resection of redundant breast skin, the modified Wise pattern produces an inverted T – shaped scar, the inferior portion of which runs along the inframammary fold (IMF) (Fig. 109-11). In an effort to eliminate the IMF scar, Lejour et al.53,54 have described a vertical scar reduction mammoplasty.
Prospective outcome analysis of women undergoing reduction mammoplasty indicate that this surgical intervention produces considerable improvements in somatic pain and in functional status.55,56 However, patients and providers also should be aware of the potential risks associated with reduction. Complications reported with these procedures include instances of nipple or skin loss, changes in levels of nipple sensation, hypertrophic scarring or keloid formation, contour deformities, and breast asymmetry.

Figure 109-9. Inferior pedicle technique for reduction mammaplasty.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



