DRUG CLASSES
Posterior pituitary hormones
• Vasopressin
Anterior pituitary hormones
• Gonadotropins
• Somatropin
• Adrenocorticotropic hormone (ACTH)
Glucocorticoids
Mineralocorticoids
PHARMACOLOGY IN PRACTICE

Janna Wong is at the clinic for a sports physical. As you measure her height and weight she tells you about a girl on the team who was sick and is now taking hormones and has grown 4 inches over the summer. Janna says that the coach favored this girl and let her use the bathroom whenever she felt like it. The classmate that Janna is describing had a pituitary tumor and needs to receive hormonal replacement for the rest of her life. Think about this situation as you read this chapter.
The pituitary gland is about the size of a green pea and lies deep within the cranial vault. The gland is not part of the brain; rather, it is suspended from the hypothalamus by the pituitary stalk and is protected by an indentation of the sphenoid bone called the sella turcica. The pituitary gland has two lobes:
• Anterior pituitary (adenohypophysis)
• Posterior pituitary (neurohypophysis)
The pituitary gland is often referred to as the “master gland” because it secretes many hormones that regulate numerous vital processes. The pituitary regulates growth, metabolism, the reproductive cycle, electrolyte balance, and water retention or loss. The hormones secreted by the anterior and posterior pituitary and the organs influenced by these hormones are shown in Figure 43.1.
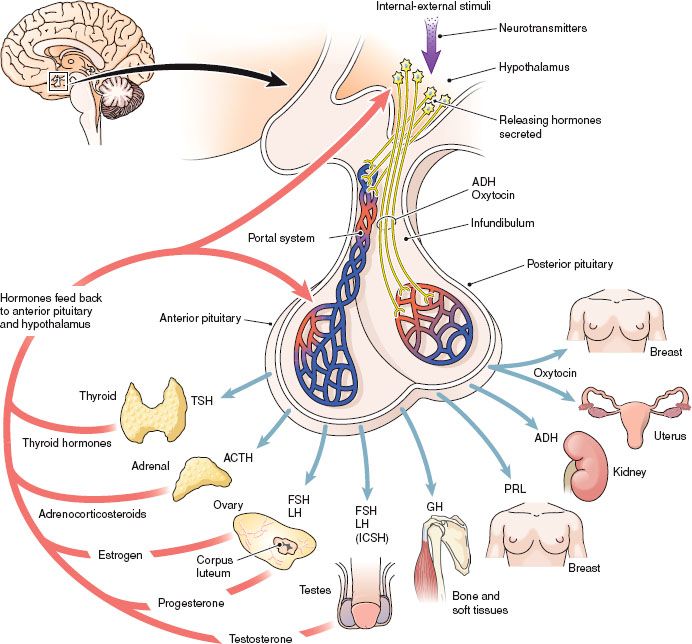
Figure 43.1 The pituitary gland and the hormones secreted by the anterior pituitary and the posterior pituitary. (From Cohen, B. J., & Taylor, J. J. [2005]. Memmler’s the human body in health and disease [10th ed.]. Baltimore: Lippincott Williams & Wilkins.)
POSTERIOR PITUITARY HORMONES
The posterior pituitary gland produces two hormones: vasopressin (antidiuretic hormone) and oxytocin (uterine stimulant). Vasopressin is discussed in this chapter and oxytocin is presented in Chapter 46.
VASOPRESSIN
Actions and Uses
Vasopressin and its derivative, desmopressin (DDAVP), regulate the reabsorption of water by the kidneys. Vasopressin is secreted by the pituitary when body fluids must be conserved. This mechanism may be activated when, for example, an individual has severe vomiting and diarrhea with little or no fluid intake. When this and similar conditions are present, the posterior pituitary releases the hormone vasopressin, water in the kidneys is reabsorbed into the blood (i.e., conserved), and the urine becomes concentrated. Vasopressin exhibits its greatest activity on the renal tubular epithelium, where it promotes water resorption and smooth muscle contraction throughout the vascular bed. Vasopressin also has some vasopressor activity.
Vasopressin and its derivative are used in treating diabetes insipidus, a disease resulting from the failure of the pituitary to secrete vasopressin or from surgical removal of the pituitary. Diabetes insipidus is characterized by a marked increase in urination (as much as 10 L in 24 hours) and excessive thirst by inadequate secretion of vasopressin (antidiuretic hormone). Treatment with vasopressin therapy replaces the hormone in the body and restores normal urination and thirst. Vasopressin may also be used for preventing and treating postoperative abdominal distention and for dispelling gas interfering with abdominal roentgenography (x-ray studies).
Adverse Reactions
Local or systemic hypersensitivity reactions may occur in some patients receiving vasopressin, and the following may also be seen:
• Tremor, sweating, vertigo
• Nasal congestion
• Nausea, vomiting, abdominal cramps
• Water intoxication (overdosage, toxicity)
Contraindications and Precautions
Vasopressin is contraindicated in patients hypersensitive to the drug or its components. Vasopressin is used cautiously in patients with a history of seizures, migraine headaches, asthma, congestive heart failure (HF), or vascular disease (because the substance may precipitate angina or myocardial infarction) and in those with perioperative polyuria. Vasopressin is classified as a pregnancy category C drug. Desmopressin acetate (a pregnancy category B drug) is typically used when diabetes insipidus occurs during pregnancy; however, it still must be used cautiously then and during lactation.
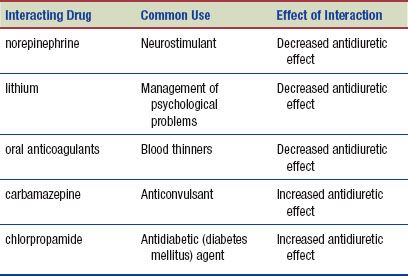
Interactions
The following interactions may occur when vasopressin is administered with another agent:
NURSING PROCESS
PATIENT RECEIVING VASOPRESSIN
ASSESSMENT
Preadministration Assessment
Patients experiencing diabetes insipidus process large amounts of fluid in their bodies. To assess drug response the weight, vital signs, and a patient history of fluid intake and output are taken. Serum electrolyte levels and other laboratory tests may be ordered by the primary health care provider.
Before administering vasopressin to relieve abdominal distention, document the patient’s blood pressure, pulse, and respiratory rate. Auscultate the abdomen and record the findings. Additionally, measure and document the patient’s abdominal girth.
Ongoing Assessment
During the ongoing assessment of a hospitalized patient, monitor the blood pressure, pulse, and respiratory rate every 4 hours or as ordered by the primary health care provider. The patient’s fluid intake and output are strictly measured. The primary health care provider is notified if there are any significant changes in these vital signs, because a dosage adjustment may be necessary.
The dosage of vasopressin or its derivatives may require periodic adjustments. After administration of the drug, observe the patient every 10 to 15 minutes for signs of an excessive dosage (e.g., blanching of the skin, abdominal cramps, and nausea). If these occur, reassure the patient that recovery from these effects will occur in a few minutes.
NURSING DIAGNOSES
Drug-specific nursing diagnoses include the following:
 Deficient Fluid Volume related to inability to replenish fluid intake secondary to diabetes insipidus
Deficient Fluid Volume related to inability to replenish fluid intake secondary to diabetes insipidus
 Acute Pain related to abdominal distention
Acute Pain related to abdominal distention
Nursing diagnoses related to drug administration are discussed in Chapter 4.
PLANNING
The expected outcomes for the patient may include an optimal response to therapy, support of patient needs related to the management of adverse reactions, and confidence in an understanding of the medication regimen.
IMPLEMENTATION
Promoting an Optimal Response to Therapy
Vasopressin may be given intramuscularly (IM) or subcutaneously (subcut) to an inpatient to treat diabetes insipidus. To prevent or relieve abdominal distention, the first dose is given 2 hours before x-ray examination and the second dose 30 minutes before the testing. An enema may be given before the first dose.
Desmopressin may be given orally, intranasally, subcut, or intravenously (IV). When the condition becomes chronic and the patient learns to self-administer the drug, adjustments are made according to the patient’s response to therapy. Patients learn to regulate their dosage based on the frequency of urination and increase of thirst. A higher dose of the drug may be taken at night to reduce thirst and urination while sleeping.
Monitoring and Managing Patient Needs
The adverse reactions associated with vasopressin, such as skin blanching, abdominal cramps, and nausea, may be decreased by administering the agent with one or two glasses of water. If these adverse reactions occur, inform the patient that they are not serious and should subside within a few minutes.
 NURSING ALERT
NURSING ALERT
Excessive dosage is manifested as water intoxication (fluid overload). Symptoms of water intoxication include drowsiness, listlessness, confusion, and headache (which may precede convulsions and coma). If signs of excessive dosage occur, notify the primary health care provider before the next dose of the drug is due; a change in the dosage, the restriction of oral or IV fluids, and the administration of a diuretic may be necessary.
DEFICIENT FLUID VOLUME. The symptoms of diabetes insipidus include the voiding of a large volume of urine at frequent intervals during the day and throughout the night. Accompanying this frequent urination is the need to drink large volumes of fluid, because patients with diabetes insipidus are continually thirsty and need to be supplied with large amounts of drinking water. Take care to refill the water container at frequent intervals. This is especially important when the patient has limited ambulatory activities. Until controlled by a drug, the symptoms of frequent urination and excessive thirst may cause a great deal of anxiety. Reassure the patient that with the proper drug therapy, these symptoms will most likely be reduced or eliminated.
Fluid intake and output are accurately measured and the patient is observed for signs of dehydration (dry mucous membranes, concentrated urine, poor skin turgor, flushing, dry skin, confusion). This is especially important early in treatment and until such time as the optimum dosage is determined and symptoms have diminished. If the patient’s output greatly exceeds intake, contact the primary health care provider. In some instances, the primary health care provider may order specific gravity and volume measurements of each voiding or at hourly intervals. Document these results in the chart to aid the primary health care provider in adjusting the dosage to the patient’s needs.
 CHRONIC CARE CONSIDERATIONS
CHRONIC CARE CONSIDERATIONS
If a person with diabetes insipidus is unable to take routine medication, a fluid deficit can rapidly occur. Therefore, individuals with diabetes insipidus should wear a medical alert bracelet so emergency personnel can be aware of this need for the medication and dosing can be continued if the patients are unable to take the drug themselves.
ACUTE PAIN. If the patient is receiving vasopressin for abdominal distention, explain the details of treating this problem and the necessity of monitoring drug effectiveness (e.g., auscultation of the abdomen for bowel sounds, insertion of a rectal tube, and measurement of the abdomen). If a rectal tube is ordered after administration of vasopressin for abdominal distention, the lubricated end of the tube is inserted past the anal sphincter and taped in place. The tube is left in place for 1 hour or as prescribed by the primary health care provider. Auscultate the abdomen every 15 to 30 minutes and measure abdominal girth hourly, or as ordered by the primary health care provider.
Educating the Patient and Family
If desmopressin is to be used nasally, ensure that the patient masters the technique of instillation. Provide illustrated patient instructions with the drug and review them with the patient. You should discuss the need to take the drug as directed by the primary health care provider. The patient should change the dosage (i.e., the prescribed number or frequency of sprays) only after consulting with the primary health care provider.
Emphasize the importance of adhering to the prescribed treatment program to control symptoms. In addition to instruction in administration, include the following in a patient and family teaching plan:
 Wear medical identification naming the disease and the drug regimen.
Wear medical identification naming the disease and the drug regimen.
 Carry a sport drink bottle to be sure to have liquids available at all times.
Carry a sport drink bottle to be sure to have liquids available at all times.
 Monitor the amount of fluids taken each day.
Monitor the amount of fluids taken each day.
 Monitor the amount and frequency of urine for each 24-hour period, reporting changes to daily patterns to your nurse.
Monitor the amount and frequency of urine for each 24-hour period, reporting changes to daily patterns to your nurse.
 Carry extra doses of the drug in case you do not make it home in time for your next dose.
Carry extra doses of the drug in case you do not make it home in time for your next dose.
 Avoid the use of alcohol while taking these drugs.
Avoid the use of alcohol while taking these drugs.
 Rotate injection sites for parenteral administration.
Rotate injection sites for parenteral administration.
 Contact the primary health care provider immediately if any of the following occurs: a significant increase or decrease in urine output, abdominal cramps, blanching of the skin, nausea, signs of inflammation or infection at the injection sites, confusion, headache, or drowsiness.
Contact the primary health care provider immediately if any of the following occurs: a significant increase or decrease in urine output, abdominal cramps, blanching of the skin, nausea, signs of inflammation or infection at the injection sites, confusion, headache, or drowsiness.
EVALUATION
 Therapeutic effect is achieved.
Therapeutic effect is achieved.
 Adverse reactions are identified, reported to the primary health care provider, and managed successfully through appropriate nursing interventions:
Adverse reactions are identified, reported to the primary health care provider, and managed successfully through appropriate nursing interventions:
• Fluid volume balance is maintained.
• Acute pain is relieved.
 Patient and family express confidence and demonstrate an understanding of the drug regimen.
Patient and family express confidence and demonstrate an understanding of the drug regimen.
ANTERIOR PITUITARY HORMONES
The hormones of the anterior pituitary include:
• Thyroid-stimulating hormone (TSH)
• Adrenocorticotropic hormone (ACTH)
• Luteinizing hormone (LH)
• Follicle-stimulating hormone (FSH)
• Growth hormone (GH)
• Prolactin
The anterior pituitary hormone TSH is discussed in Chapter 44. The remaining hormones are covered in this chapter and can be classified as follows:
- ACTH is produced by the anterior pituitary and stimulates the adrenal cortex to secrete the corticosteroids in response to biological stress.
- FSH and LH are called gonadotropins because they influence the gonads (the organs of reproduction).
- GH, also called somatropin, contributes to the growth of the body during childhood, especially the growth of muscles and bones.
- Prolactin, which is also secreted by the anterior pituitary, stimulates the production of breast milk in the postpartum patient. Additional functions of prolactin are not well understood. Prolactin is the only anterior pituitary hormone that is not used medically.
GONADOTROPINS: FOLLICLE-STIMULATING HORMONE AND LUTEINIZING HORMONE
The gonadotropins (FSH and LH) influence the secretion of sex hormones, the development of secondary sex characteristics, and the reproductive cycle in both men and women.
Action and Uses
These drugs are purified preparations of the gonadotropins (FSH and LH) extracted from the urine of postmenopausal women or produced by a recombinant form of DNA. Gonadotropins are used to induce ovulation and pregnancy in anovulatory women (women whose bodies fail to produce an ovum or fail to ovulate). Menopur is also used in assisted reproductive technology (ART) programs to stimulate multiple follicles for in vitro fertilization. Besides their use in treating female infertility, some of these drugs are used in men. Human chorionic gonadotropin (HCG) is extracted from human placentas. The actions of HCG are identical to those of the pituitary LH. This drug is also used in boys to treat prepubertal cryptorchism (failure of the testes to descend into the scrotum) and in men to treat selected cases of hypogonadotropic hypogonadism. Follistim AQ is used to induce sperm production (spermatogenesis). For additional information on the gonadotropins, see the Summary Drug Table: Pituitary and Adrenocortical Hormones.
Clomiphene and ganirelix are synthetic nonsteroidal compounds that bind to estrogen receptors, decreasing the amount of available estrogen receptors and causing the anterior pituitary to increase secretion of FSH and LH. These drugs are used to induce ovulation in anovulatory (nonovulating) women.
Adverse Reactions
Hormone-Associated Reactions
• Vasomotor flushes (which are like the hot flashes of menopause)
• Breast tenderness
• Abdominal discomfort, ovarian enlargement
• Hemoperitoneum (blood in the peritoneal cavity)
Generalized Reactions
• Nausea, vomiting
• Headache, irritability, restlessness, fatigue
• Edema and irritation at the injection site
 LIFESPAN CONSIDERATIONS
LIFESPAN CONSIDERATIONS
Childbearing Women
Fetal effects have been demonstrated in animal studies when gonadotropins have been administered. Birth defects have been reported in human studies; therefore, gonadotropins should not be administered to women known to be pregnant.
Contraindications, Precautions, and Interactions
These drugs are contraindicated in patients who are hypersensitive to the drug or any component of the drug. The gonadotropins are contraindicated in patients with high gonadotropin levels, thyroid dysfunction, adrenal dysfunction, liver disease, abnormal bleeding, ovarian cysts, or sex hormone–dependent tumors, or those with an organic intracranial lesion (pituitary tumor). Gonadotropins are contraindicated during pregnancy (pregnancy category X).
These drugs are used cautiously in patients with epilepsy, migraine headaches, asthma, or cardiac or renal dysfunction, and during lactation. There are no known clinically significant interactions with the gonadotropins.
NURSING PROCESS
PATIENT RECEIVING VASOPRESSIN
ASSESSMENT
Preadministration Assessment
These drugs are almost always administered on an outpatient basis and may be self-administered by the patient. Before prescribing any of these drugs, the primary health care provider takes a thorough medical history and performs a physical examination. Additional laboratory and diagnostic tests for ovarian function and tubal patency may also be performed. A pelvic examination may be performed by the primary health care provider to rule out ovarian enlargement, pregnancy, or uterine problems.
Ongoing Assessment
At the time of each office or clinic visit, ask the patient about the occurrence of adverse reactions and document the patient’s vital signs and weight.
NURSING DIAGNOSES
Drug-specific nursing diagnoses include the following:
 Acute Pain related to adverse reactions (ovarian enlargement, irritation at the injection site)
Acute Pain related to adverse reactions (ovarian enlargement, irritation at the injection site)
 Anxiety related to inability to conceive, treatment outcome, other factors
Anxiety related to inability to conceive, treatment outcome, other factors
Nursing diagnoses related to drug administration are discussed in Chapter 4.
PLANNING
The expected outcomes of the patient may include an optimal response to drug therapy, support of patient needs related to the management of adverse reactions, reduction in anxiety, and confidence in an understanding of the medication regimen.
IMPLEMENTATION
Promoting an Optimal Response to Therapy
Gonadotropin injections are given in the primary health care provider’s office or the clinic or may be self-administered. These drugs must be administered IM or subcut because they are destroyed in the gastrointestinal (GI) tract; therefore, they cannot be taken orally. Injection sites are rotated and previous sites are checked for redness and irritation.
 NURSING ALERT
NURSING ALERT
If the patient complains of visual disturbances, the drug therapy is discontinued and the primary health care provider notified. An examination by an ophthalmologist is usually indicated.
Monitoring and Managing Patient Needs
Acute Pain. Female patients taking these drugs are usually examined by the primary health care provider frequently to detect excessive ovarian stimulation, called hyperstimulation syndrome (sudden ovarian enlargement with ascites). The patient may or may not report pain. This syndrome usually develops quickly, within 3 to 4 days.
 NURSING ALERT
NURSING ALERT
The patient is checked for signs of excessive ovarian enlargement (abdominal distention, pain, ascites [with serious cases]). The drug is discontinued at the first sign of ovarian stimulation or enlargement. The patient is usually admitted to the hospital for supportive measures.
ANXIETY. Patients seeking treatment to become pregnant often experience a great deal of anxiety due to past failed attempts. In addition, when taking these drugs, the patient faces the possibility of multiple births. The success rate of these drugs varies and depends on many factors. The primary health care provider usually discusses the value of this, as well as other approaches, with the patient and her sexual partner. Allow the patient time to talk about her concerns about the proposed treatment program.
Educating the Patient and Family
Patients are instructed by the primary health care provider about the frequency of sexual intercourse. You can assess whether the patient understands the directions given by the primary health care provider. When a gonadotropin is prescribed, you may instruct the patient how to use the device to inject the hormone and to keep all primary health care provider appointments and to report adverse reactions to the nurse or primary health care provider. Include the following information when a gonadotropin is prescribed:
HORMONAL OVARIAN STIMULANTS
 Before beginning therapy, be aware of the possibility of multiple births and birth defects.
Before beginning therapy, be aware of the possibility of multiple births and birth defects.
 It is a good idea to use a calendar to track the treatment schedule and ovulation.
It is a good idea to use a calendar to track the treatment schedule and ovulation.
 Report bloating, abdominal pain, flushing, breast tenderness, and pain at the injection site.
Report bloating, abdominal pain, flushing, breast tenderness, and pain at the injection site.
NONHORMONAL OVARIAN STIMULANTS
 Take the drug as prescribed (5 days) and do not stop taking the drug before the course of therapy is finished unless told to do so by the primary health care provider.
Take the drug as prescribed (5 days) and do not stop taking the drug before the course of therapy is finished unless told to do so by the primary health care provider.
 Notify the primary health care provider if bloating, stomach or pelvic pain, jaundice, blurred vision, hot flashes, breast discomfort, headache, nausea, or vomiting occurs.
Notify the primary health care provider if bloating, stomach or pelvic pain, jaundice, blurred vision, hot flashes, breast discomfort, headache, nausea, or vomiting occurs.
 Keep in mind that if ovulation does not occur after the first course of therapy, a second or third course may be used. If therapy does not succeed after three courses, the drug is considered unsuccessful and is discontinued.
Keep in mind that if ovulation does not occur after the first course of therapy, a second or third course may be used. If therapy does not succeed after three courses, the drug is considered unsuccessful and is discontinued.
EVALUATION
 Therapeutic effect is achieved.
Therapeutic effect is achieved.
 Adverse reactions are identified, reported to the primary health care provider, and managed successfully through appropriate nursing interventions:
Adverse reactions are identified, reported to the primary health care provider, and managed successfully through appropriate nursing interventions:
• Patient is free of pain.
• Anxiety is managed successfully.
 Patient expresses confidence and demonstrates an understanding of the drug regimen.
Patient expresses confidence and demonstrates an understanding of the drug regimen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


