12
Pharmacotherapy of the Epilepsies
This chapter will be most useful after having a basic understanding of the material in Chapter 21, Pharmacotherapy of the Epilepsies in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition includes:
• A detailed discussion of the nature and mechanisms of seizures, including models of epilepsy
• A discussion of the genetic approaches to the epilepsies
• The structures of the antiseizure drugs
• A detailed discussion of the general principles that determine the choice of drugs for therapy of the epilepsies
LEARNING OBJECTIVES
 Know the different seizure types and the mechanisms that are responsible for each type.
Know the different seizure types and the mechanisms that are responsible for each type.
 Understand the mechanisms of action of the antiseizure drugs and how they are used to treat the different types of seizures.
Understand the mechanisms of action of the antiseizure drugs and how they are used to treat the different types of seizures.
 Describe the toxicities, adverse effects, and salient drug interactions of drugs used to treat the epilepsies.
Describe the toxicities, adverse effects, and salient drug interactions of drugs used to treat the epilepsies.
 Know the general principles of drug therapy of the epilepsies.
Know the general principles of drug therapy of the epilepsies.
DRUGS INCLUDED IN THIS CHAPTER
• Acetazolamide (DIAMOX, others)
• Carbamezepine (TEGRETOL, CARBATROL, others)
• Clonazepam (KLONOPIN, others)
• Clorazepate (TRANXENE, others)
• Ethosuximide (ZARONTIN, others)
• Felbamate (FELBATOL)—withdrawn from market
• Fosphenytoin (CEREBYX, others)
• Gabapentin (NEURONTIN, others)
• Lacosamide (VIMPAT)
• Lamotrigine (LAMICTAL, others)
• Levetiracetam (KEPPRA, others)
• Methsuximide (CELONTIN)
• Oxcarbazepine (TRILEPTAL, others)
• Phenobarbital (LUMINAL, others)
• Phenytoin (DILANTIN, others)
• Pregabalin (LYROCA, others)
• Rufinamide (BANZEL)
• Tiagabine (GABITRIL)
• Topiramate (TOPAMAX, others)
• Valproic acid (DEPAKENE, others)
• Vigabatrin (SABRIL)
• Zonisamide (ZONEGRAN, others)
MECHANISMS OF ACTION OF ANTISEIZURE DRUGSa
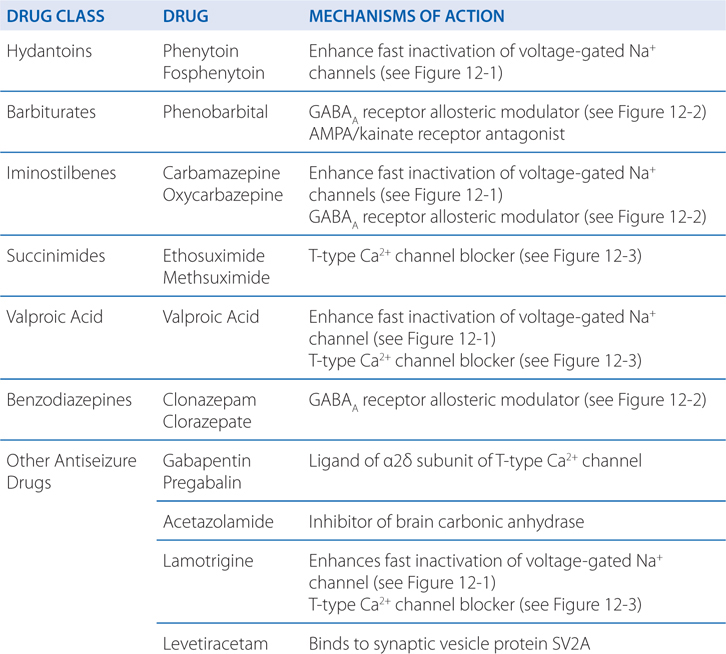
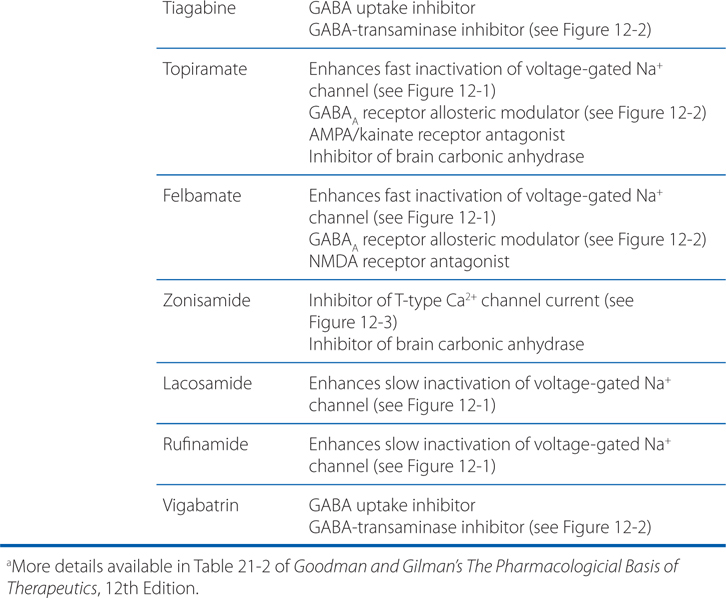
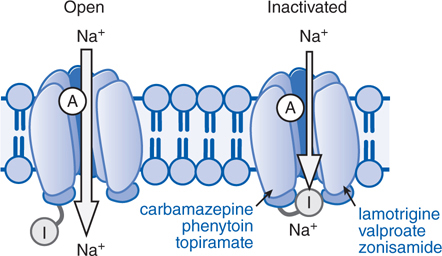
• Limit the sustained, repetitive firing of voltage-activated Na+ channels (see Figure 12-1)
FIGURE 12-1 Antiseizure drug-enhanced Na+ channel inactivation. Some antiseizure drugs (shown in blue text) prolong the inactivation of the Na+ channels, thereby reducing the ability of neurons to fire at high frequencies. Note that the inactivated channel itself appears to remain open, but is blocked by the inactivation gate (I). A, activation gate.
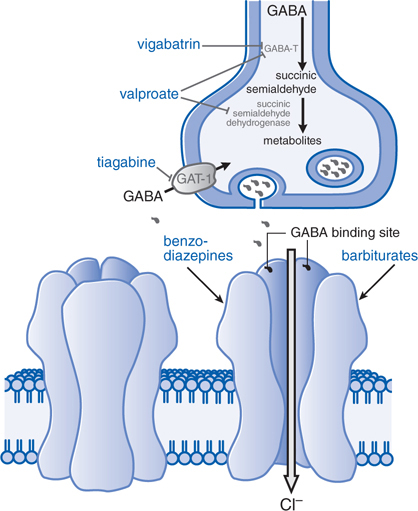
• Enhanced γ-aminobutyric acid (GABA)-mediated synaptic inhibition either by a presynaptic or postsynaptic action (see Figure 12-2)
FIGURE 12-2 Enhanced GABA synaptic transmission. In the presence of GABA, the GABAA receptor (structure on left) is opened, allowing an influx of C1–, which in turn increases membrane polarization. Some antiseizure drugs (show in larger blue text) act by reducing the metabolism of GABA. Others act at the GABAA receptor, enhancing C1– influx in response to GABA. Gabapentin acts presynaptically to promote GABA release; its molecular target is currently under investigation. GABA molecules, GABA-T, GABA transaminase; GAT-1, GABA transporter.
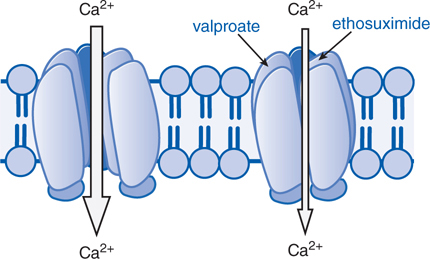
• Inhibition of voltage-activated Ca2+ channels responsible for T-type Ca2+ currents (see Figure 12-3)
FIGURE 12-3 Antiseizure drug-induced reduction of current through T-type Ca2+ channels. Some antiseizure drugs (shown in blue text) reduce the flow of Ca2+ through T-type Ca2+ channels thus reducing the pacemaker current that underlies the thalamic rhythm in spikes and waves seen in generalized absence seizures.
• Early diagnosis and treatment of seizure disorders with a single appropriate agent offers the best prospect of achieving prolonged seizure-free periods with the lowest risk of toxicity.
• The efficacy combined with the unwanted effects of a given drug determine which particular drug is optimal for a given patient.
• Unless extenuating circumstances such as status epilepticus exist, only monotherapy should be initiated.
• Dosage is increased at appropriate intervals as required for control of seizures or as limited by toxicity.
• If compliance has been confirmed yet seizures persist, another drug should be substituted.
• If therapy with a second single drug is inadequate, combination therapy is warranted.
• It is wise to select 2 drugs that each act by distinct mechanisms.
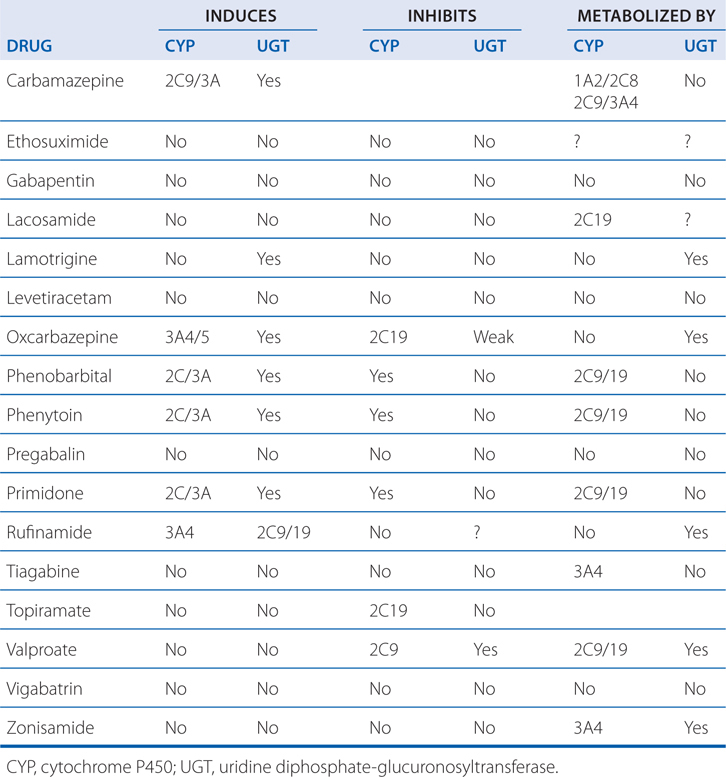
• Side effects of each drug and the potential drug interactions also should be considered when using 2 drugs (see Table 12-1).
TABLE 12-1 Interactions of Anti-Seizure Drugs with Hepatic Microsomal Enzymes
• Measurement of plasma drug concentration at appropriate intervals greatly facilitates the initial adjustment of dosage to minimize dose-related adverse effects without sacrificing seizure control.
• Once initiated, antiseizure drugs are typically continued for at least 2 years.
• When discontinuing, medications should be tapered slowly over a period of several months.
A 12-year-old girl has her first tonic-clonic seizure while at school. Her seizure was preceded by lip smacking and lasted about 1 minute during which she lost consciousness.
a. During subsequent neurological tests, her parents ask for a classification of epileptic seizures so that they might understand where their child falls in the spectrum of epilepsies. What is a useful classification of epilepsies?
Table 12-2 classifies epileptic seizures into partial seizures and generalized seizures. The type of epileptic seizure is one determinant of the drug selected for therapy.
TABLE 12-2 Classification of Epileptic Seizures
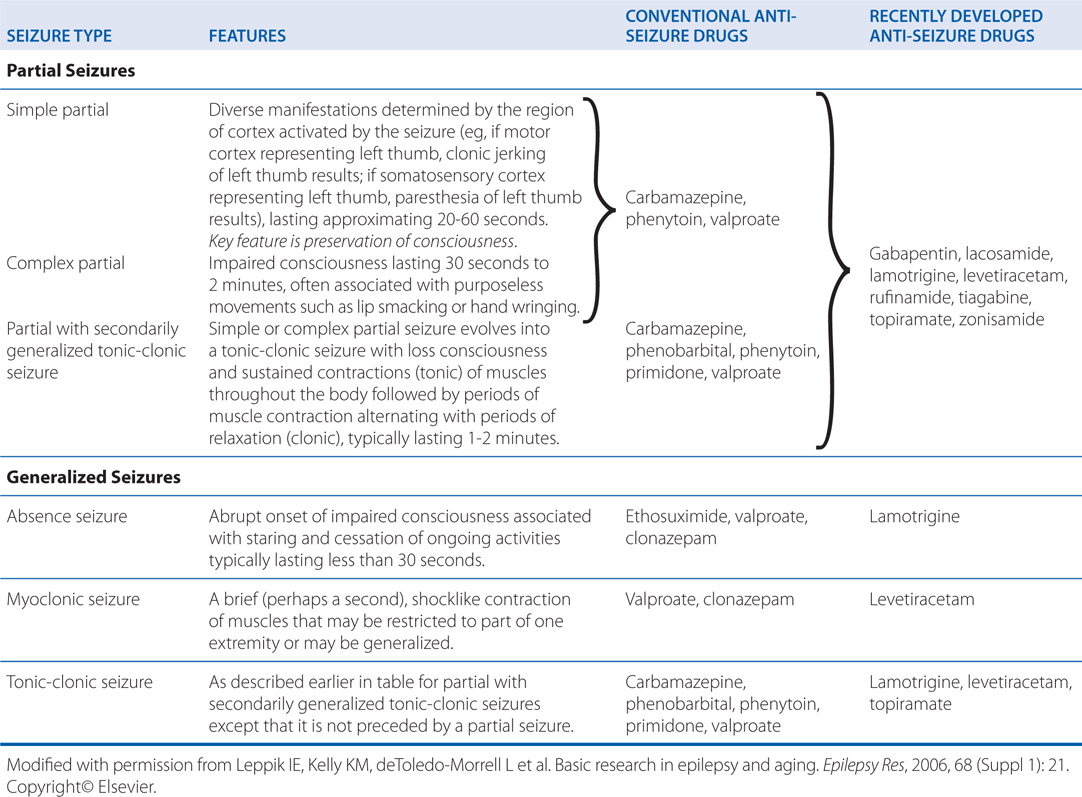
Apart from this epileptic seizure classification, an additional classification specifies epileptic syndromes, which refer to a cluster of symptoms frequently occurring together and include seizure types, etiology, age of onset, and other factors. More than 50 distinct epileptic syndromes have been identified and categorized into partial versus generalized epilepsies. The partial epilepsies may consist of any of the partial seizure types (see Table 12-2) and account for roughly 60% of all epilepsies. The etiology commonly consists of a lesion in some part of the cortex, such as a tumor, developmental malformation, or damage due to trauma or stroke. Such lesions often are evident on brain magnetic resonance imaging (MRI). Alternatively, the etiology may be genetic.
The generalized epilepsies are characterized most commonly by one or more of the generalized seizure types listed in Table 12-2 and account for ~40% of all epilepsies. The etiology is usually genetic. The most common generalized epilepsy is referred to as juvenile myoclonic epilepsy, accounting for ~10% of all epileptic syndromes. The age of onset is in the early teens, and the condition is characterized by myoclonic, tonic-clonic, and often absence seizures. Like most of the generalized-onset epilepsies, juvenile myoclonic epilepsy is a complex genetic disorder that is probably due to inheritance of multiple susceptibility genes; there is a familial clustering of cases, but the pattern of inheritance is not mendelian. The classification of epileptic syndromes guides clinical assessment and management, and in some instances, selection of antiseizure drugs.
b. What seizure type is this patient exhibiting?
Although it is difficult to make an absolute diagnosis without all of the clinical data, it would seem that this patient has had a complex partial seizure with a secondarily generalized tonic-clonic seizure.
c. Should antiseizure medication be started immediately in this patient?
Early diagnosis and treatment of seizure disorders with a single appropriate agent offers the best prospect of achieving prolonged seizure-free periods with the lowest risk of toxicity. An attempt should be made to determine the cause of the epilepsy with the hope of discovering a correctable lesion, either structural or metabolic. The efficacy combined with the unwanted effects of a given drug determines which particular drug is optimal for a given patient.
The first decision to make is whether and when to initiate treatment. For example, it may not be necessary to initiate antiseizure therapy after an isolated tonic-clonic seizure in a healthy young adult who lacks a family history of epilepsy and who has a normal neurological examination, a normal EEG, and a normal brain MRI scan. The odds of seizure recurrence in the next year (15%) are similar to the risk of a drug reaction sufficiently severe to warrant discontinuation of medication. On the other hand, a similar seizure occurring in an individual with a positive family history of epilepsy, an abnormal neurological examination, an abnormal EEG, and an abnormal MRI carries a risk of recurrence approximating 60%, odds that favor initiation of therapy.
d. What other general principles of the treatment of seizure disorders should be followed in this patient?
The general principles of therapy of the epilepsies are outlined in the Side Bar GENERAL PRINCIPLES AND CHOICE OF DRUGS FOR THE THERAPY OF THE EPILEPSIES.
The patient in Case 12-1 had a second tonic-clonic seizure and it was decided that she should be treated with phenytoin.
a. What is the mechanism of action of phenytoin?
The mechanisms of action of antiseizure drugs are listed in the Table MECHANISMS OF ACTION OF ANTISEIZURE DRUGS. Phenytoin limits the repetitive firing of action potentials evoked by a sustained depolarization of mouse spinal cord neurons maintained in vitro. This effect is mediated by a slowing of the rate of recovery of voltage-activated Na+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree