8
Psychopharmacology
Chapter 8 Psychopharmacology is a combination of Chapter 15, Drug Therapy of Depression and Anxiety Disorders and Chapter 16, Pharmacology of Psychosis and Mania from Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. An understanding of the material in those chapters will be helpful in following the material presented in this chapter. In addition to the material presented here, the above chapters in the 12th Edition include:
• A characterization of depressive and anxiety disorders
• Table 15-2, The Potencies of Antidepressants at the Human Transporters for Norepinephrine (NET), Serotonin (SERT), and Dopamine (DAT)
• Table 15-4, Potencies of Selected Antidepressants at Muscarinic, Histamine H1, and alpha1-Adrenergic Receptors
• A discussion of the long-term adaptive effects of antidepressants that enhance the effectiveness of therapy
• A discussion of the pathophysiology of psychosis
• A discussion of the chemistry of antipsychotic agents
• The role of dopamine and serotonin receptors in antipsychotic therapy
• A detailed discussion of the use antipsychotic agents for nonpsychotic disorders
• A discussion of the adverse effects of antipsychotic agents that are not predicted by monoamine receptor affinities
• Novel treatments for psychosis and mania
LEARNING OBJECTIVES
 Understand the mechanisms of action, therapeutic uses, and adverse effects of antidepressant drugs.
Understand the mechanisms of action, therapeutic uses, and adverse effects of antidepressant drugs.
 Know how antidepressant drugs are used to manage depression.
Know how antidepressant drugs are used to manage depression.
 Know the mechanisms of action, therapeutic uses, and adverse effects of antipsychotic drugs and drugs used to treat mania.
Know the mechanisms of action, therapeutic uses, and adverse effects of antipsychotic drugs and drugs used to treat mania.
 Understand the pharmacotherapy of acute and chronic psychoses and bipolar disorder.
Understand the pharmacotherapy of acute and chronic psychoses and bipolar disorder.
DRUGS INCLUDED IN THIS CHAPTER
• Amitriptyline (ELAVIL, others)
• Amoxapine (ASENDIN)
• Aripiprazole (ABILIFY)
• Asenapine (SAPHRIS, others)
• Atomoxetine (STRATTERA)
• Bupropion (WELLBUTRIN, ZYBAN, others)
• Buspirone (BUSPAR)
• Carbamazepine (TEGRETOL, others)
• Chlorpromazine (THORAZINE, others)
• Citalopram (CELEXA)
• Clomipramine (ANAFRANIL)
• Clozapine (CLOZARIL, others)
• Desipramine (NORPRAMIN)
• Desvenlafaxine (PRISTIQ)
• Doxepin (ADAPIN, SINEQUAN)
• Droperidol (INAPSIN, others)
• Duloxetine (CYMBALTA)
• Escitalopram (LEXAPRO)
• Fluoxetine (PROSAC, SYMBYAX, others)
• Fluphenazine (PROLIXIN, others)
• Fluvoxamine (LUVOX)
• Haloperidol (HALDOL, others)
• Iloperidone (FANAPT)
• Imipramine (TOFRANIL, others)
• Isocarboxazid (MARPLAN)
• Lamotrigine (LAMICTAL)
• Lithium
• Loxapine (LOXITANE)
• Maprotiline (LUDIOMIL)
• Mianserin (DEPNON, others)—not approved in the United States
• Milnacipran (IXEL, others)—not approved in the United States
• Mirtazapine (REMERON, others)
• Molindone (MOBAN)—use discontinued
• Nefazodone (DUTONIN, others)
• Nortriptyline (PAMELOR)
• Olanzapine (ZYPREXA)
• Paliperidone (INVEGA)
• Paroxetine (PAXIL)
• Perphenazine (TRILAFON, others)
• Phenelzine (NARDIL)
• Protriptyline (VIVACTIL)
• Quetiapine (SEROQUEL)
• Reboxetine (EDRONAX)—not available in the United States
• Risperidone (RESPERDAL)
• Selegiline (EMSAM)
• Sertindole (SERDOLECT, others)—not approved in the United States
• Sertraline (ZOLOFT)
• Tranylcypromine (PARNATE)
• Trazodone (DESYREL)
• Trifluoperazine (STELAZINE, others)
• Trimipramine (SURMONTIL)
• Valproic acid, divalproex sodium (DEPAKENE, DEPAKOTE, others)
• Venlafaxine (EFFEXOR)
• Ziprasidone (GEODON)
MECHANISMS OF ACTION OF PSYCHOPHARMACOLOGICAL AGENTS
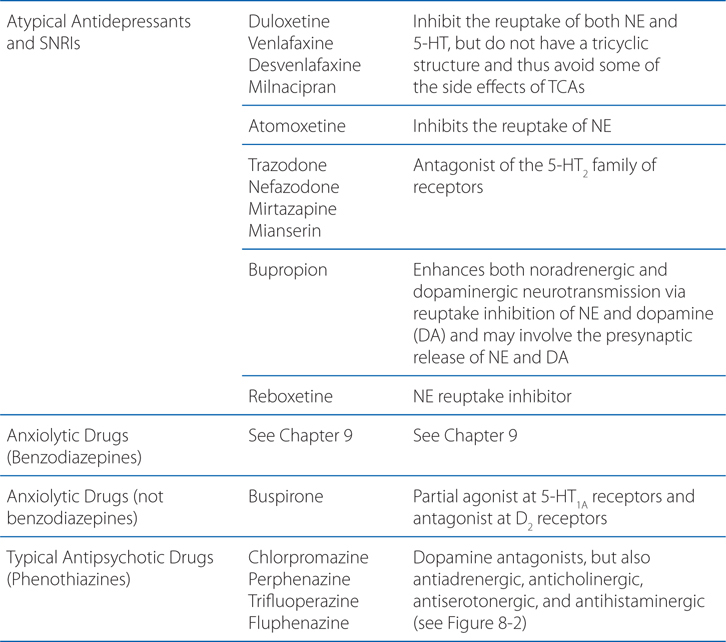
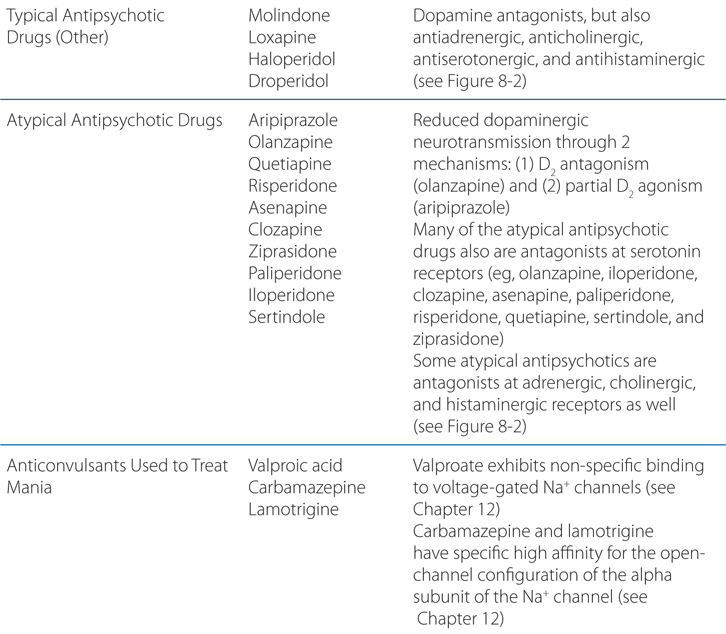
A 39-year-old woman is taking amitriptyline for major depression.
a. How do antidepressants act to elevate the mood of patients?
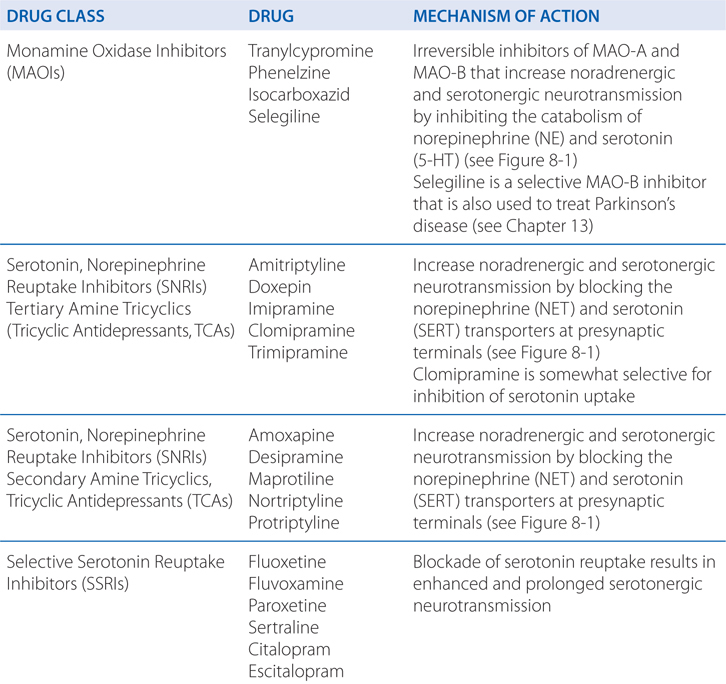
Many different antidepressants have established track records of efficacy for treating major depression. However, they all suffer some limitations in efficacy, since at least 20% of all depressed patients are refractory to multiple different antidepressants at adequate doses. In monoamine systems, reuptake of the transmitter is the main mechanism by which neurotransmission is terminated; thus, inhibition of reuptake can enhance neurotransmission, presumably by slowing clearance of the transmitter from the synapse and prolonging the dwell-time of the transmitter in the synapse. Enhancing neurotransmission may subsequently lead to adaptive changes (see Figure 8-1).
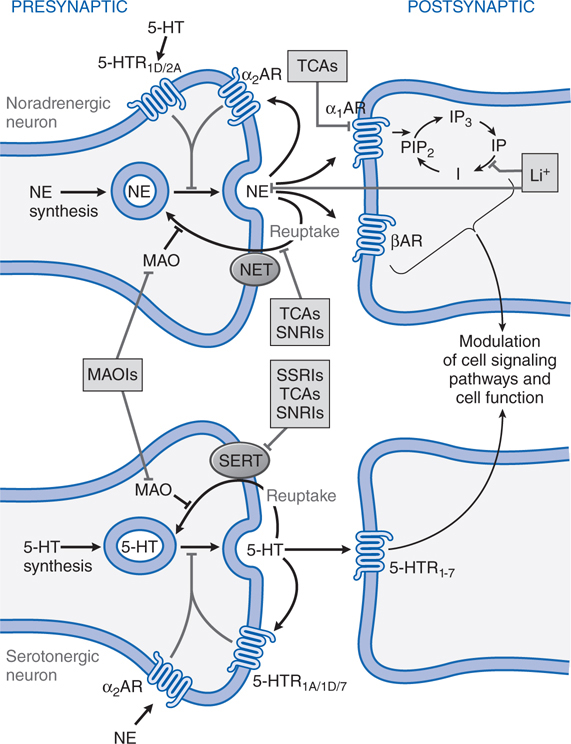
FIGURE 8-1 Sites of action of antidepressants. Schematics representing noradrenergic (top) and serotonergic (bottom) nerve terminals. SSRIs, SNRIs, and TCAs increase noradrenergic or serotonergic neurotransmission by blocking the norepinephrine or serotonergic transporter at presynaptic terminals (NET, SERT). MAOIs inhibit the catabolism of norepinephrine and serotonin. Some antidepressants such as trazodone and related drugs have direct effects on serotonergic receptors that contribute to their clinical effects. Chronic treatment with a number of antidepressants desensitizes presynaptic autoreceptors and heteroreceptors, producing long-lasting changes in monoaminergic neurotransmission. Post-receptor effects of antidepressant treatment, including modulation of GPCR signaling and activation of protein kinases and ion channels, are involved in the mediation of the long-term effects of antidepressant drugs. Note that NE and 5-HT also affect each other’s neurons.
Long-term effects of antidepressant drugs evoke adaptive or regulatory mechanisms that enhance the effectiveness of therapy. These responses include increased adrenergic or serotonergic receptor density or sensitivity, increased receptor-G protein coupling and cyclic nucleotide signaling, induction of neurotrophic factors, and increased neurogenesis in the hippocampus. Persistent antidepressant effects depend on the continued inhibition of 5-HT or NE transporters, or enhanced serotonergic or noradrenergic neurotransmission achieved by an alternative pharmacological mechanism. For example, chronic treatment with some antidepressants that interact directly with monoamine transporters (eg, SSRIs, SNRIs, or NE reuptake inhibitors) reduces the expression and activity of 5-HT or NE transporters in the brain, which results in enhanced serotonergic or noradrenergic neurotransmission.
b. What are the major considerations when starting an antidepressant medication?
Following initiation of antidepressant drug treatment, there is generally a “therapeutic lag” lasting 3 to 4 weeks before a measurable therapeutic response becomes evident. This is the reason that electroconvulsive therapy may be the treatment of choice for agitated, depressed patients with a high risk of suicide. Some patients may respond to antidepressant treatment sooner than 3 to 4 weeks; others may require more than 8 weeks for an adequate response. In general, if a patient does not respond to a given antidepressant after an 8-week trial on an adequate dose, then switching to another antidepressant with a different mechanism of action is a reasonable next step (eg, SSRI to SNRI). If a partial response has been observed, other drugs may be added to the primary SSRI or SNRI medications; these additive medications include the antidepressant drug bupropion, thyroid hormone (triiodothyronine), or an atypical antipsychotic (aripiprazole or olanzapine).
After the successful initial treatment phase, a 6- to 12-month maintenance treatment phase is typical, after which the drug is gradually withdrawn. If a patient has experienced 2 separate episodes of major depression or is chronically depressed (ie, >2 years), lifelong treatment with an antidepressant is advisable.
A controversial issue regarding the use of all antidepressants is their relationship to suicide. Data establishing a clear link between antidepressant treatment and suicide are lacking. The FDA has issued a “black box” warning regarding the use of SSRIs and a number of other antidepressants in children and adolescents, particularly during the early phase of treatment, due to the possibility of an association between antidepressant treatment and suicide.
c. What are the adverse effects that this patient might expect?
Amitriptyline is a tricyclic antidepressant. TCAs are potent anticholinergics and antagonists at histamine H1 receptors; H1 receptor antagonism contributes to the sedative effects of TCAs (Table 8-1). Antagonism of muscarinic acetylcholine receptors contributes to cognitive dulling as well as a range of adverse effects mediated by the parasympathetic nervous system (blurred vision, dry mouth, tachycardia, constipation, difficulty urinating). Some tolerance does occur for these anticholinergic effects, which are mitigated by titration strategies to reach therapeutic doses over a reasonable period of time. Antagonism of α1-adrenergic receptors contributes to orthostatic hypotension and sedation. Weight gain is another side effect of this class of antidepressants.
TABLE 8-1 Antidepressants: Chemical Structures, Dose and Dosage Forms, and Side Effects
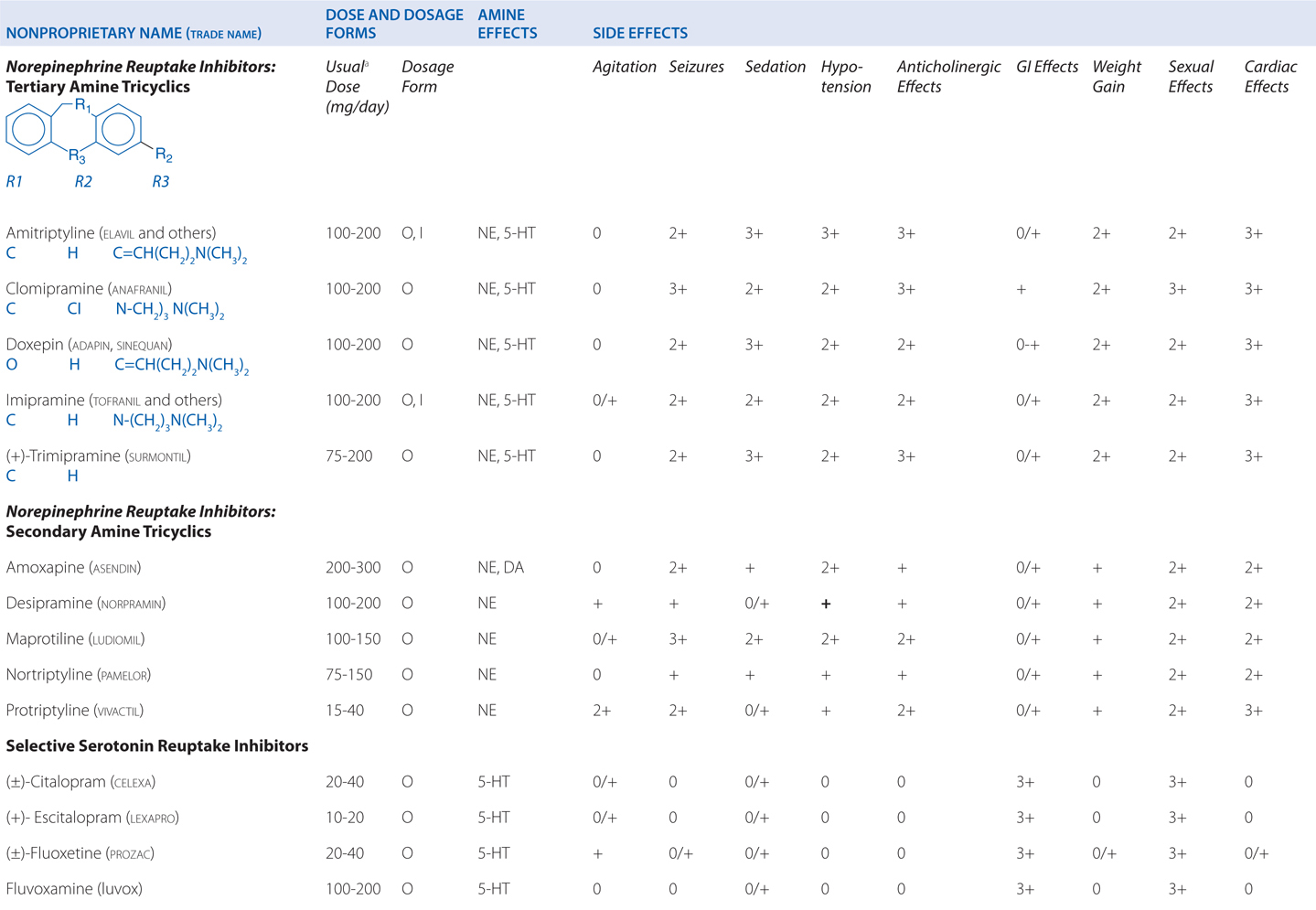
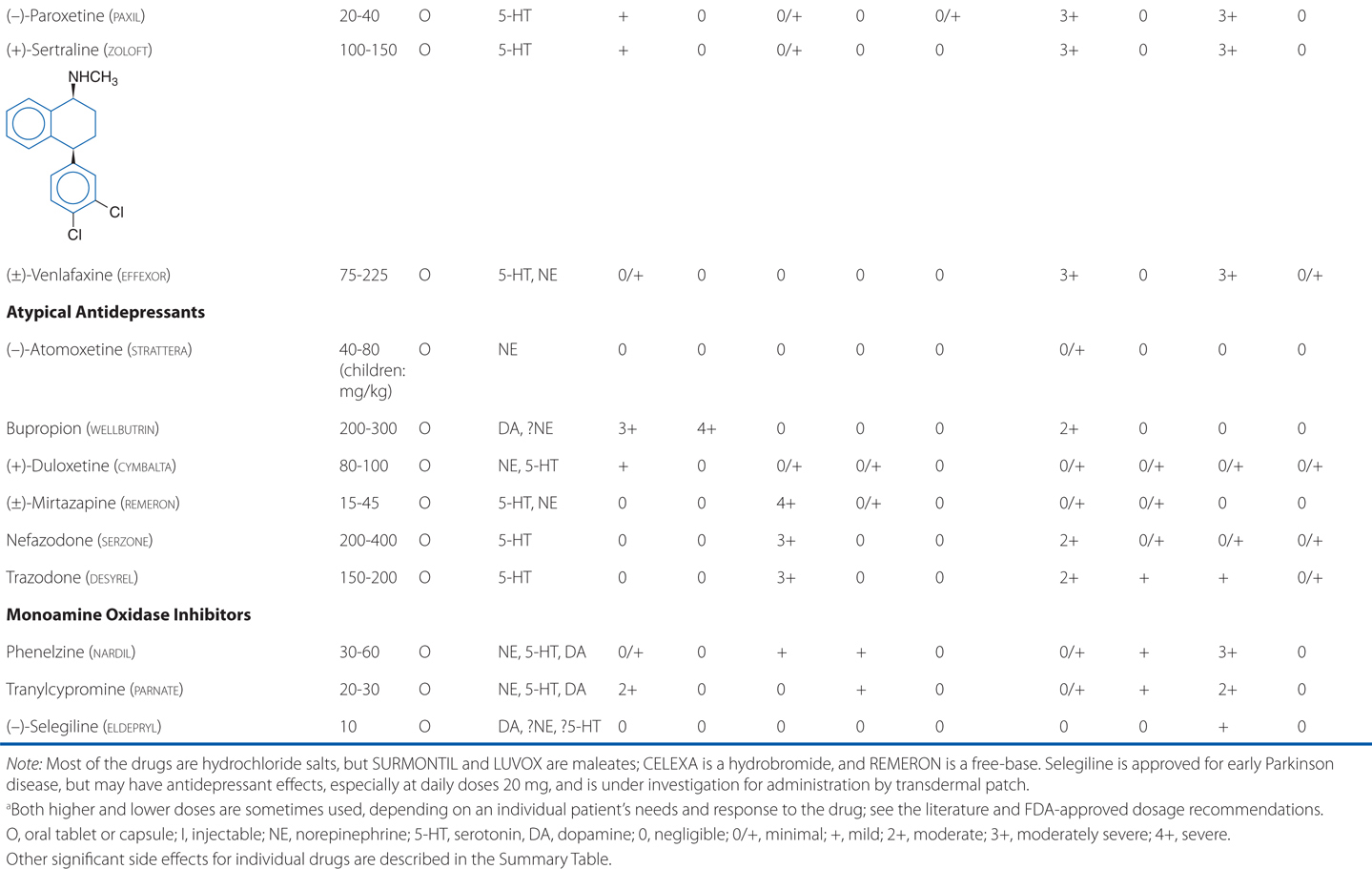
TCAs also have quinidine-like effects on cardiac conduction that can be life-threatening with overdose and limit the use of TCAs in patients with coronary heart disease. This is the primary reason that no more than a 1-week supply should be provided to a new patient; even during maintenance treatment, only a very limited supply should be available to the patient at any given time. Like other antidepressant drugs, TCAs also lower the seizure threshold.
d. What are this patient’s options if the side effects of amitriptyline become intolerable?
The SSRIs are also effective in treating major depression. All of the SSRIs show a clear improvement in safety margin compared to the TCAs and are much safer in overdose, and in clinical practice have affected a broad range of psychiatric, behavioral, and medical conditions, for which they are used, on- and off-label.
In addition to use as antidepressants, SSRIs also are anxiolytics with demonstrated efficacy in the treatment of generalized anxiety, panic, social anxiety, and obsessive-compulsive disorders.
A 48-year-old man has developed depression after the death of his wife. He is prescribed venlafaxine.
a. How does venlafaxine differ from other antidepressants?
Many older TCAs block both SERT and NET, but with a high side effect burden. Four medications with a nontricyclic structure that inhibit the reuptake of both 5-HT and norepinephrine have been approved for use in the United States for treatment of depression, anxiety disorders, and pain: venlafaxine and its demethylated metabolite, desvenlafaxine; duloxetine; and milnacipran (approved only for fibromyalgia pain in the United States). The rationale behind the development of these newer agents was that targeting both SERT and NET, analogous to the effects of some TCAs, might improve overall treatment response.
b. Why is there a delay in the onset of antidepressant effect with SSRI antidepressants and SNRIs such as venlafaxine?
SNRIs inhibit both SERT and NET. Depending on the drug, the dose, and the potency at each site, SNRIs cause enhanced serotonergic and/or noradrenergic neurotransmission. Similar to the action of SSRIs, the initial inhibition of SERT induces activation of 5-HT1A and 5-HT1D autoreceptors. This action decreases serotonergic neurotransmission by a negative feedback mechanism until these serotonergic autoreceptors are desensitized. Then, the enhanced serotonin concentration in the synapse can interact with postsynaptic 5-HT receptors.
c. What side effects might this patient expect with venlafaxine?
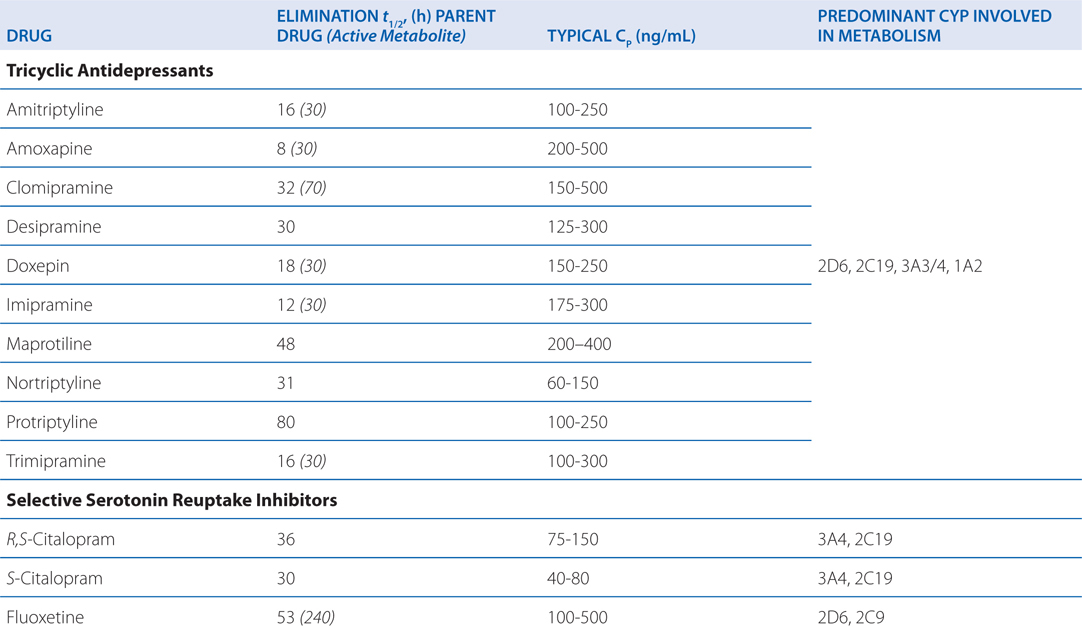
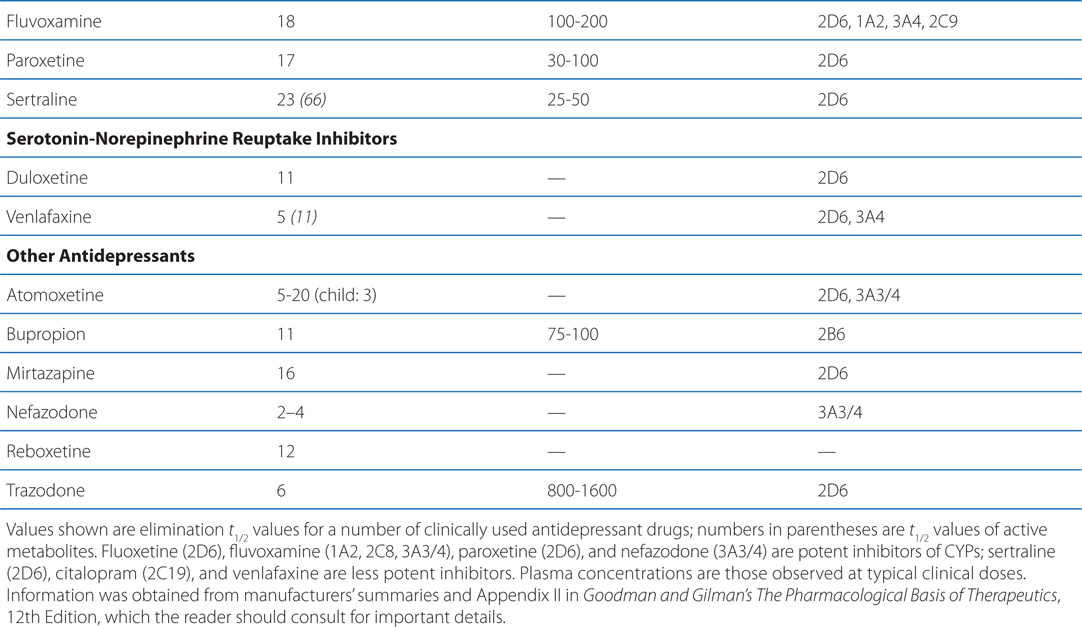
The side effects of venlafaxine are shown in Table 8-1. Venlafaxine dose reductions are suggested for patients with renal or hepatic impairment (see Table 8-2). The SNRIs have desirable safety advantages over the TCAs. SNRIs have a side effect profile similar to that of the SSRIs, including nausea, constipation, insomnia, headaches, and sexual dysfunction. The immediate release formulation of venlafaxine can induce sustained diastolic hypertension (systolic blood pressure > 90 mm Hg at consecutive weekly visits) in 10 to 15% of patients at higher doses; this risk is reduced with the extended-release form.
TABLE 8-2 Disposition of Antidepressants
A 53-year-old man with mild depression has been treated with a monoamine oxidase inhibitor. His physician is now switching his antidepressant therapy to fluoxetine.
a. What drug interactions should he be cautioned about while taking the SSRI?
Most antidepressants, including the SSRIs, exhibit drug—drug interactions based on their routes of metabolism (see Table 8-2). Paroxetine and, to a lesser degree, fluoxetine are potent inhibitors of CYP2D6. The other SSRIs, outside of fluvoxamine, are at least moderate inhibitors of CYP2D6. This inhibition can result in disproportionate increases in plasma concentrations of drugs metabolized by CYP2D6 when doses of these drugs are increased. Fluvoxamine directly inhibits CYP1A2 and CYP2C19; fluoxetine and fluvoxamine also inhibit CYP3A4. See Chapter 2 for a list of drugs metabolized by these CYPs.
b. He had previously been taking tranylcypromine, an MAO inhibitor. What concerns are there for switching his medication to an SSRI?
Another important drug–drug interaction with SSRIs occurs via a pharmacodynamic mechanism. MAOIs enhance the effects of SSRIs due to inhibition of serotonin metabolism. Administration of these drugs together can produce synergistic increases in extracellular brain serotonin, leading to the serotonin syndrome. Symptoms of the serotonin syndrome include hyperthermia, muscle rigidity, myoclonus, tremors, autonomic instability, confusion, irritability, and agitation; this can progress toward coma and death. Other drugs that may induce the serotonin syndrome include substituted amphetamines such as methylenedioxymethamphetamine (Ecstasy), which directly releases serotonin from nerve terminals. The primary treatment is stopping all serotonergic drugs, administering nonselective serotonin antagonists, and supportive measures.
Since currently available MAOIs bind irreversibly to MAO and block the enzymatic metabolism of monoaminergic neurotransmitters, SSRIs should not be started until at least 14 days following discontinuation of treatment with an MAOI; this allows for synthesis of new MAO. For all SSRIs but fluoxetine, at least 14 days should pass prior to beginning treatment with an MAOI following the end of treatment with an SSRI. Since the active metabolite of fluoxetine, norfluoxetine, has a t1/2 of 1 to 2 weeks, at least 5 weeks should pass between stopping fluoxetine and beginning an MAOI.
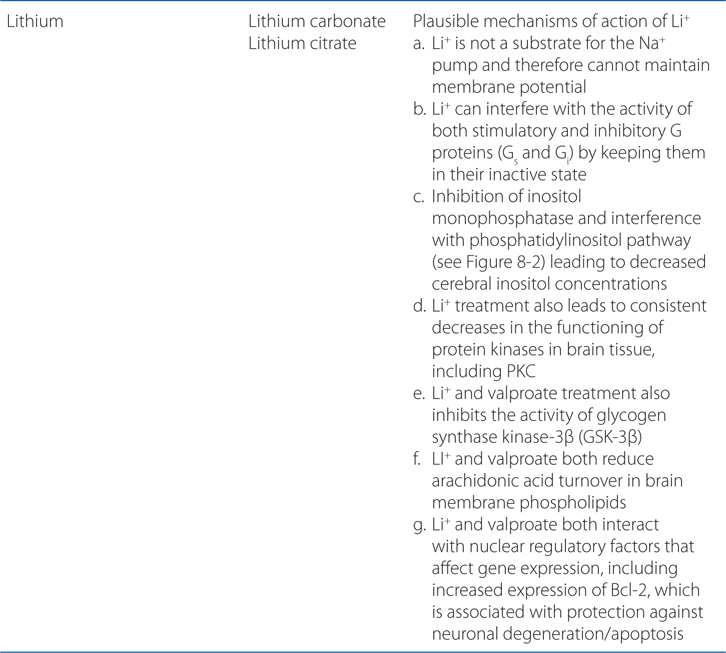
A 49-year-old man is in hospital for surgery on a herniated vertebral disc. On the second day following surgery, he becomes agitated, belligerent, paranoid, and aggressive. He is given haloperidol to control his behavior.
a. What kind of drug is haloperidol and how does it act?
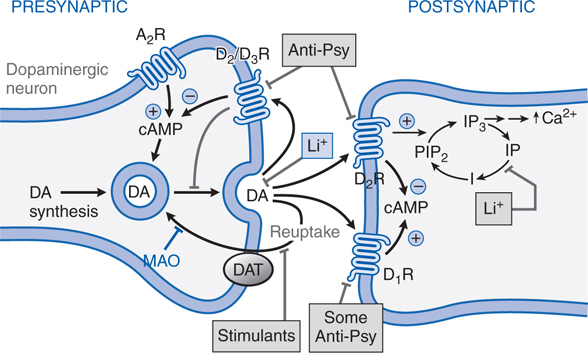
Like most antipsychotic drugs haloperidol is an antagonist at D2 receptors (see Figure 8-2; Tables 8-3 and 8-4).
FIGURE 8-2 Sites of action of antipsychotic agents and Li+. In varicosities (“terminals”) along terminal arborizations of dopaminergic neurons projecting from midbrain to forebrain, DA is synthesized and stored in vesicles. Following exocytotic release, DA interacts with postsynaptic receptors (R) of D1 and D2 types, and presynaptic D2 and D3 autoreceptors. Termination of DA action occurs primarily by active transport of DA into presynaptic terminals via the DA transporter DAT, with secondary deamination by mitochondrial monoamine oxidase (MAO). Stimulation of postsynaptic D1 receptors activates the Gs-adenylyl cyclase-cAMP pathway. D2 receptors couple through Gi to inhibit adenylyl cyclase and through Gq to activate the PLC-IP3-Ca2+ pathway. Activation of the Gi pathway can also activate K+ channels, leading to hyperpolarization. Lithium inhibits the phosphatase that liberates inositol (I) from inositol phosphate (IP). Li+ can also inhibit depolarization-evoked release of DA and NE, but not 5-HT. D2-like autoreceptors suppress synthesis of DA by diminishing phosphorylation of rate-limiting TH, and by limiting DA release. In contrast, presynaptic A2 adenosine receptors (A2R) activate AC and, through cyclic AMP production, TH activity. All antipsychotic agents act at D2 receptors and autoreceptors; some also block D1 receptors. Stimulant agents inhibit DA reuptake by DAT, thereby prolonging the dwell time of synaptic DA. Initially in antipsychotic treatment, DA neurons release more DA, but following repeated treatment, they enter a state of physiological depolarization inactivation, with diminished production and release of DA, in addition to continued receptor blockade. (“T-line”), inhibition or blockade; +, elevation of activity; –, reduction of activity.
TABLE 8-3 Chemical Structures, Dosages for Acute Psychosis and Schizophrenia Maintenance, and Metabolic Risk Profilea
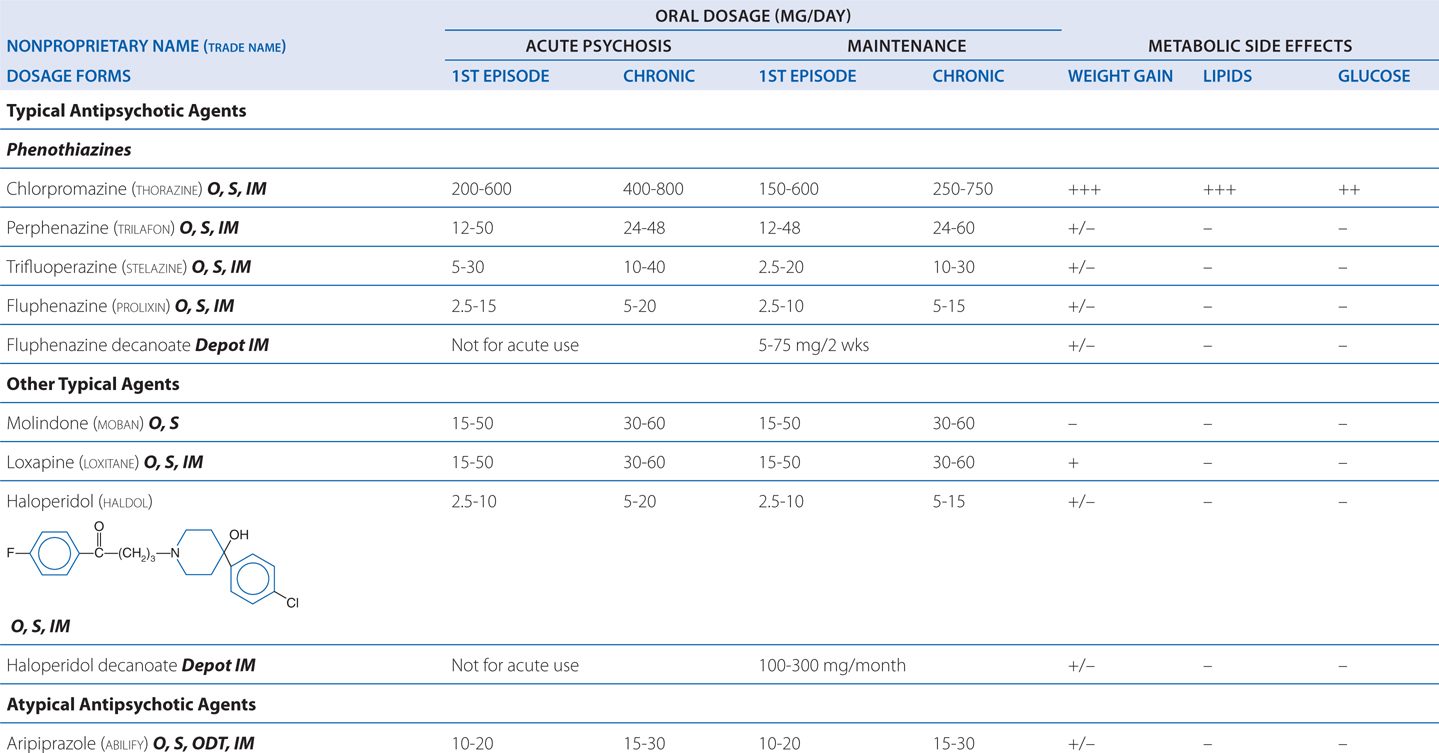
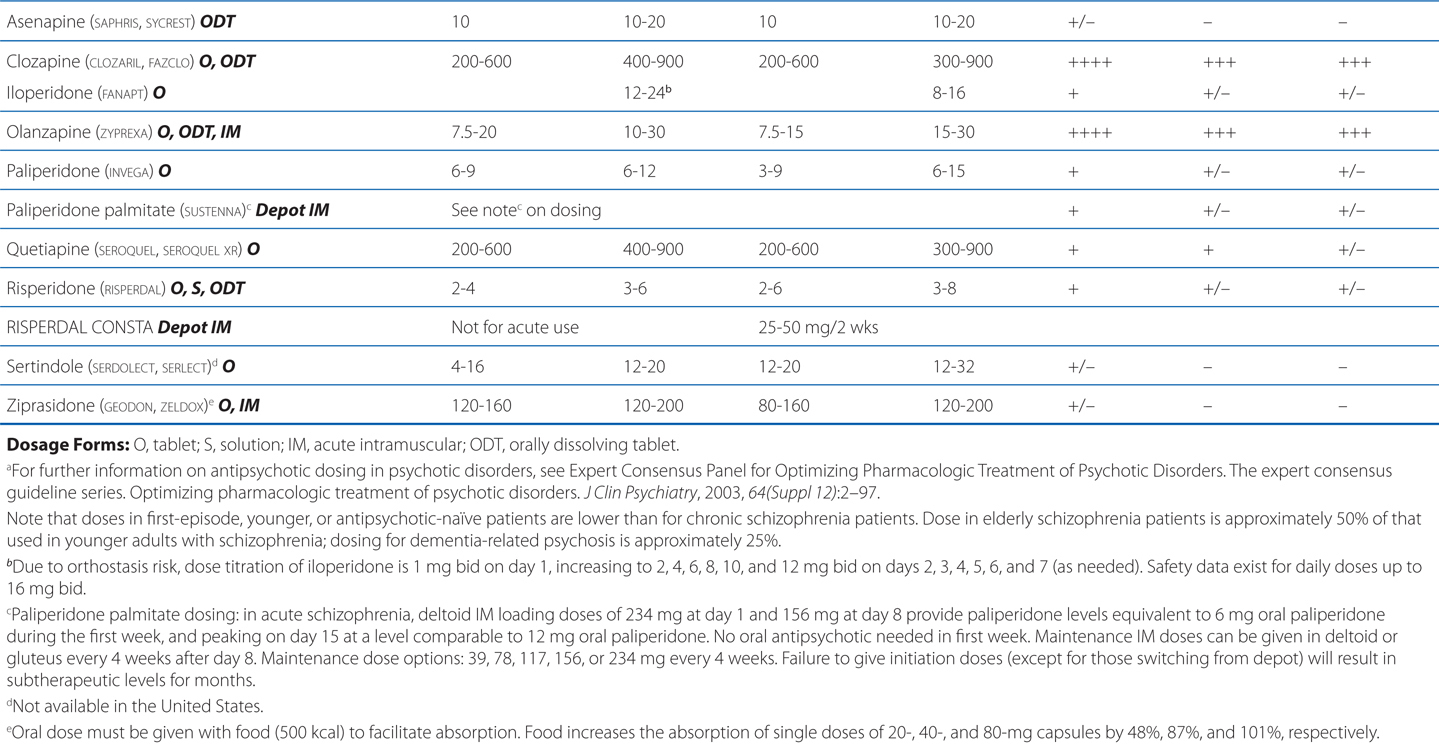
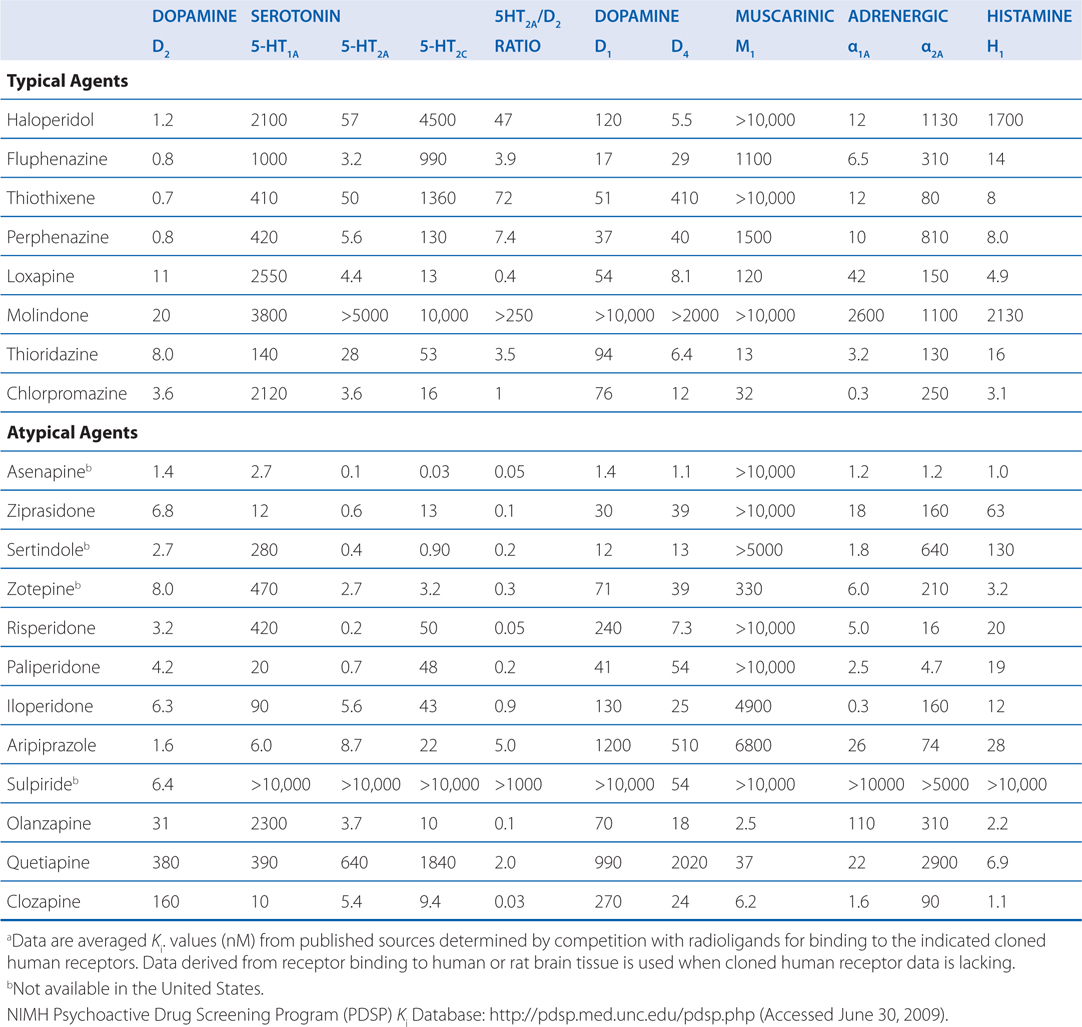
TABLE 8-4 Potencies of Antipsychotic Agents at Neurotransmitter Receptorsa
b. What are the advantages of using a drug such as haloperidol in this setting?
Delirium following surgery generally requires only short-term therapy. Because anticholinergic drug effects may worsen delirium and dementia, high-potency typical antipsychotic drugs (eg, haloperidol) or atypical antipsychotic agents with limited antimuscarinic properties (eg, risperidone) are often the drugs of choice.
c. While not a consideration in this setting, what are the side effects of the chronic use of haloperidol?
Excessive D2 blockade, as is often the case with the use of high-potency typical agents (eg, haloperidol), not only increases risk for motor neurological effects (eg, muscular rigidity, bradykinesia, tremor, akathisia) (see Figure 8-3), but also slows mentation (bradyphrenia), and interferes with central reward pathways, resulting in patient complaints of anhedonia. Rarely used are low-potency typical agents (eg, chlorpromazine), which also have high affinities for H1, M, and α1 receptors that cause many undesirable effects (sedation, anticholinergic properties, orthostasis). Concerns regarding QTc prolongation (eg, thioridazine) further limit their clinical usefulness.
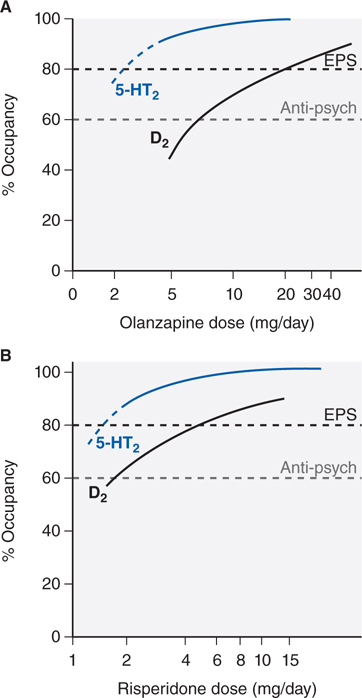
FIGURE 8-3 Receptor occupancy and clinical response for antipsychotic agents. Typically, in D2 receptor occupancy by the drug more than 60% provides antipsychotic effects, receptor occupancy more than 80% causes extrapyramidal symptoms (EPS). Atypical agents combine weak D2 receptor blockade with more potent 5-HT2A antagonism/inverse agonism. Inverse agonism at 5-HT2 receptor subtypes may contribute to the reduced EPS risk of olanzapine (Panel A) and risperidone (Panel B) and efficacy at lower D2 receptor occupancy (olanzapine, Panel A). Aripiprazole is a partial D2 agonist that can achieve only 75% functional blockade.
d. What are other options in treating this patient?
Intramuscular (IM) administration of ziprasidone, aripiprazole, or olanzapine represents an option for treating agitated and minimally cooperative patients, and presents less risk of drug-induced parkinsonism than haloperidol. QTc prolongation associated with intramuscular droperidol and intravenous administration of haloperidol have curtailed the use of those particular formulations. Treatment continues until agitated or hallucinatory behaviors are controlled and the underlying etiologies are addressed.
A 32-year-old woman with a long history of drug abuse has been diagnosed with schizophrenia.
a. What are the goals of short-term therapy with this patient?
The immediate goals of acute antipsychotic treatment are the reduction of agitated, disorganized, or hostile behavior, decreasing the impact of hallucinations, the improvement of organization of thought processes, and the reduction of social withdrawal. Doses used are often higher than those required for maintenance treatment of stable patients.
b. What are the goals of long-term therapy with this patient?
The need for long-term treatment poses issues almost exclusively to the chronic psychotic illnesses, schizophrenia and schizoaffective disorder, although long-term antipsychotic treatment is sometimes used for manic patients, for ongoing psychosis in dementia patients, for L-dopa psychosis, and for adjunctive use in SSRI-unresponsive major depression.
The choice of antipsychotic agents for long-term schizophrenia treatment is based primarily on avoidance of adverse effects and, when available, prior history of patient response. Since schizophrenia spectrum disorders are lifelong diseases, treatment acceptability is paramount to effective illness management. Whether atypical antipsychotic agents are superior to typical antipsychotic agents has been the subject of significant and contentious debate.
c. She is prescribed the atypical antipsychotic drug aripiprazole. What is the mechanism of action of aripiprazole?
Presently, antipsychotic agents include many different chemical structures with a range of activities at different neurotransmitter receptors (eg, 5-HT2A antagonism, 5-HT1A partial agonism). As a result, structure-function relationships that were relied upon in the past have become less important. Instead, receptor-function relationships and functional assays are more clinically relevant. Aripiprazole represents a good example of how an examination of the structure provides little insight into its mechanism, which is based on dopamine receptor partial agonism. Detailed knowledge of receptor affinities (see Table 8-4) and the functional effect at specific receptors (eg, full, partial, or inverse agonism or antagonism) can provide important insight into the therapeutic and adverse effects of antipsychotic agents.
USE OF ANTIPSYCHOTIC AGENTS IN NONPSYCHOTIC DISORDERS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree