Pharmacotherapy of Psychosis and Mania
Psychosis is a symptom of mental illnesses characterized by a distorted or nonexistent sense of reality. Common psychotic disorders include mood disorders (major depression or mania) with psychotic features, substance-induced psychosis, dementia with psychotic features, delirium with psychotic features, brief psychotic disorder, delusional disorder, schizoaffective disorder, and schizophrenia. Schizophrenia has a worldwide prevalence of 1%, but patients with schizophrenia exhibit features that extend beyond those seen in other psychotic illnesses. The positive symptoms of psychotic disorders include: hallucinations, delusions, disorganized speech, and disorganized or agitated behavior. Schizophrenia patients also suffer from negative symptoms (apathy, avolition, alogia), and cognitive deficits, particularly deficits in working memory, processing speed, and social cognition.
The dopamine (DA) hypothesis of psychosis was derived from the discovery that chlorpromazine and reserpine exhibited therapeutic antipsychotic properties in schizophrenia by decreasing dopaminergic neurotransmission. The DA overactivity hypothesis led to the development of the first therapeutic class of antipsychotic agents, now referred to as typical or first-generation antipsychotic drugs. The term “neuroleptic” refers to typical antipsychotic drugs that act through D2 receptor blockade but are associated with extrapyramidal side effects.
The DA hypothesis has its limitations: it does not account for the cognitive deficits associated with schizophrenia and does not explain the psychotomimetic effects of LSD (e.g., d-lysergic acid, a potent serotonin 5HT2 receptor agonist) or the effects of phencyclidine and ketamine, antagonists of the N-methyl-D-aspartate (NMDA) glutamate receptor. Advances in treatment have emerged from exploration of alternative (non-dopaminergic) mechanisms for psychosis and from experience with atypical antipsychotic agents such as clozapine. The newer atypical antipsychotics potently antagonize the 5HT2 receptor, while blocking D2 receptors less potently than older typical antipsychotic agents, resulting in antipsychotic efficacy with limited extrapyramidal side effects. Promising medications target glutamate and 5HT7 receptor subtypes, receptors for γ-aminobutyric acid (GABA) and acetylcholine (both muscarinic and nicotinic), and peptide hormone receptors (e.g., oxytocin).
PHARMACOTHERAPY
The 12th edition of the parent text reviews the relevant pathophysiology and the general goals of pharmacotherapy of psychosis and mania. Regardless of the underlying pathology, the immediate goal of antipsychotic treatment is a decrease in the acute symptoms that induce patient distress, particularly behavioral symptoms (e.g., hostility, agitation) that may present a danger to the patient or others. The dosing, route of administration, and choice of antipsychotic depend on the underlying disease state, clinical acuity, drug-drug interactions with concomitant medications, and patient sensitivity to short- or long-term adverse effects. With the exception of clozapine’s superior efficacy in treatment-refractory schizophrenia, neither the clinical presentation nor biomarkers predict the likelihood of response to a specific antipsychotic class or agent. As a result, avoidance of adverse effects based upon patient and drug characteristics and exploitation of certain medication properties (e.g., sedation related to histamine H1 or muscarinic antagonism) are the principal determinants for choosing initial antipsychotic therapy.
All commercially available antipsychotic drugs reduce dopaminergic neurotransmission (Figure 16–1). Chlorpromazine and other early low-potency typical antipsychotic agents are also profoundly sedating, a feature that used to be considered relevant to their therapeutic pharmacology. The development of the high-potency typical antipsychotic agent haloperidol, a drug with limited H1 and M1 affinity and significantly less sedative effect, demonstrate that sedation is not necessary for antipsychotic activity, although at times desirable.
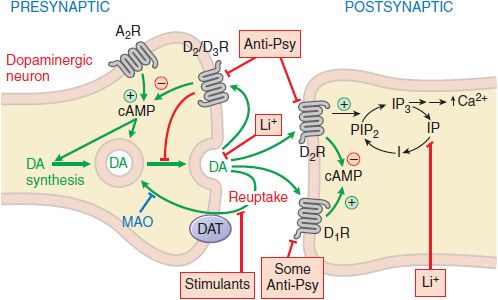
Figure 16–1 Sites of action of antipsychotic agents and Li+. Following exocytotic release, DA interacts with both postsynaptic receptors and presynaptic autoreceptors. Termination of DA action occurs primarily by reuptake into presynaptic terminals via the DA transporter DAT, with secondary deamination by mitochondrial monoamine oxidase (MAO). Stimulation of postsynaptic D1 receptors activates the Gs-adenylyl cyclase-cAMP pathway. D2 receptors couple through Gi to inhibit adenylyl cyclase and through Gq to activate the PLC-IP3-Ca2+ pathway. Activation of the Gi pathway can also activate K+ channels, leading to hyperpolarization. Li+ inhibits the phosphatase that liberates inositol (I) from inositol phosphate (IP). Li+ can also inhibit depolarization-evoked release of DA and NE, but not 5HT. D2-like autoreceptors suppress synthesis of DA by diminishing phosphorylation of rate-limiting tyrosine hydroxylase (TH), and by limiting DA release. In contrast, presynaptic A2 adenosine receptors (A2R) activate the AC-cAMP-PKA pathway, thereby enhancing TH activity. All antipsychotic agents act at D2 receptors and autoreceptors; some also block D1 receptors (see Table 16–2). Stimulant agents inhibit DA reuptake by DAT, thereby prolonging the dwell time of synaptic DA. Initially in antipsychotic treatment, DA neurons release more DA, but following repeated treatment, they enter a state of physiological depolarization inactivation, with diminished production and release of DA, in addition to continued receptor blockade.  , inhibition or blockade; +, elevation of activity; –, reduction of activity.
, inhibition or blockade; +, elevation of activity; –, reduction of activity.
SHORT-TERM TREATMENT
DELIRIUM AND DEMENTIA. Disease variables have considerable influence on selection of antipsychotic agents. Psychotic symptoms of delirium or dementia are generally treated with low medication doses, although doses may have to be repeated at frequent intervals initially to achieve adequate behavioral control. Despite widespread clinical use, not a single antipsychotic drug has received approval for dementia-related psychosis. Moreover, all antipsychotic drugs carry warnings that they may increase mortality in this setting. Because anticholinergic drug effects may worsen delirium and dementia, high-potency typical antipsychotic drugs (e.g., haloperidol) or atypical antipsychotic agents with limited antimuscarinic properties (e.g., risperidone) are often the drugs of choice.
The best tolerated doses in dementia patients are one-fourth of adult schizophrenia doses. Extrapyramidal neurological symptoms (EPSs), orthostasis, and sedation are particularly problematic in this patient population (see Chapter 22). Significant antipsychotic benefits are usually seen in acute psychosis within 60-120 min after drug administration. Oral dissolving tablet (ODT) preparations for risperidone, aripiprazole, and olanzapine, or liquid concentrate forms of risperidone or aripiprazole, are options for some patients. The dissolving tablets adhere to any moist tongue or oral surface, cannot be spit out, and are then swallowed along with oral secretions. Intramuscular (IM) administration of ziprasidone, aripiprazole, or olanzapine represents an option for treating agitated and minimally cooperative patients, and presents less risk for drug-induced parkinsonism than haloperidol. QTc prolongation associated with intramuscular droperidol and intravenous administration of haloperidol have curtailed use of those particular formulations.
MANIA. All atypical antipsychotic agents with the exception of clozapine and iloperidone have indications for acute mania, and doses are titrated rapidly to the maximum recommended dose over the first 24-72 h of treatment. Acute mania patients with psychosis require very high daily doses. Clinical response (decreased psychomotor agitation and irritability, increased sleep, and reduced or absent delusions and hallucinations) usually occurs within 7 days. Patients with mania may need to continue on antipsychotic treatment for many months after the resolution of psychotic and manic symptoms, typically in combination with a mood stabilizer such as lithium or valproic acid preparations. Combining an antipsychotic agent with a mood stabilizer often improves control of manic symptoms, and further reduces the risk of relapse. Weight gain from the additive effects of antipsychotic agents and mood stabilizers (lithium, valproic acid) presents a significant clinical problem.
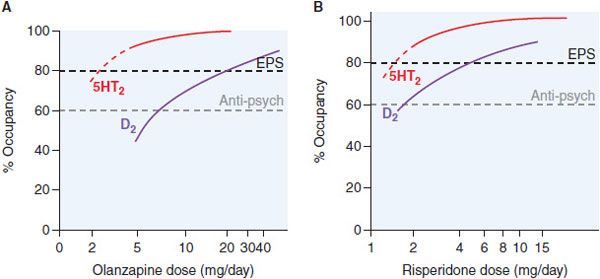
MAJOR DEPRESSION. Patients with major depressive disorder with psychotic features require lower than average doses of antipsychotic drugs, given in combination with an antidepressant. Most antipsychotic drugs show limited antidepressant benefit as monotherapy agents. However, atypical antipsychotic agents are efficacious as adjunct therapy in treatment-resistant depression. Their clinical efficacy may be related to the fact that almost all atypical antipsychotic medications are potent 5HT2A antagonists (Figure 16–2).
Figure 16–2 Receptor occupancy and clinical response for antipsychotic agents. Typically, D2 receptor occupancy by the drug >60% provides antipsychotic effects; receptor occupancy >80% causes extrapyramidal symptoms (EPS). Atypical agents combine weak D2 receptor blockade with more potent 5HT2A antagonism/inverse agonism. Inverse agonism at 5HT2 receptor subtypes may contribute to the reduced EPS risk of olanzapine (Panel A) and risperidone (Panel B) and efficacy at lower D2 receptor occupancy (olanzapine, Panel A). Aripiprazole is a partial D2 agonist that can achieve only 75% functional blockade.
5HT2A and 5HT2C antagonism facilitates DA release and increases noradrenergic outflow from the locus coeruleus. Administration of 5HT2A and 5HT2C antagonists in the form of low doses of atypical antipsychotic agents, along with selective serotonin reuptake inhibitors (SSRIs), increases responses rates in SSRI nonresponders. A combination preparation of low-dose olanzapine and fluoxetine is approved for bipolar depression, and low-dose risperidone (i.e., 1 mg) increases clinical response rates when added to existing SSRI treatment in SSRI nonresponders. Aripiprazole is FDA-approved for adjunctive use in antidepressant nonresponders, again at low doses (2-15 mg). Aripiprazole and most other antipsychotic drugs are ineffective as monotherapy for bipolar depression, with quetiapine being the sole exception.
SCHIZOPHRENIA. Newer, atypical antipsychotic agents offer a better neurological side-effect profile than typical antipsychotic drugs. Atypical agents show markedly reduced EPS risk compared to typical antipsychotic agents. Excessive D2 blockade increases risk for motor neurological effects (e.g., muscular rigidity, bradykinesia, tremor, akathisia), slows mentation (bradyphrenia), and interferes with central reward pathways, resulting in patient complaints of anhedonia. In acute psychosis, sedation may be desirable, but the use of a sedating antipsychotic drug may interfere with a patient’s cognitive function and social reintegration.
Schizophrenia patients have a 2-fold higher prevalence of metabolic syndrome and type 2 diabetes mellitus (DM) and twice the rate of cardiovascular (CV) related mortality than the general population. Consensus guidelines recommend baseline determination of serum glucose, lipids, weight, blood pressure, and when possible, waist circumference and personal and family histories of metabolic and CV disease. Drug-induced parkinsonism can also occur, especially among elderly patients exposed to antipsychotic agents that have high D2 affinity (e.g., typical antipsychotic drugs); recommended doses are ~50% of those used in younger schizophrenia patients.
LONG-TERM TREATMENT
The choice of antipsychotic agents for long-term schizophrenia treatment is based primarily on avoidance of adverse effects and, when available, prior history of patient response. Because schizophrenia spectrum disorders are lifelong diseases, treatment acceptability is paramount to effective illness management. Atypical antipsychotic agents offer significant advantages related to reduced neurological risk, with long-term tardive dyskinesia rates <1%, or approximately one-fifth to one-tenth of that seen with typical antipsychotic drugs.
Antipsychotic treatments are associated with metabolic risks that include: weight gain, dyslipidemia (particularly hypertriglyceridemia), an adverse impact on glucose-insulin homeostasis, including new-onset type 2 DM, and diabetic ketoacidosis (DKA), with reported fatalities from the latter. Clozapine and olanzapine have the highest metabolic risk and are only used as last resort.
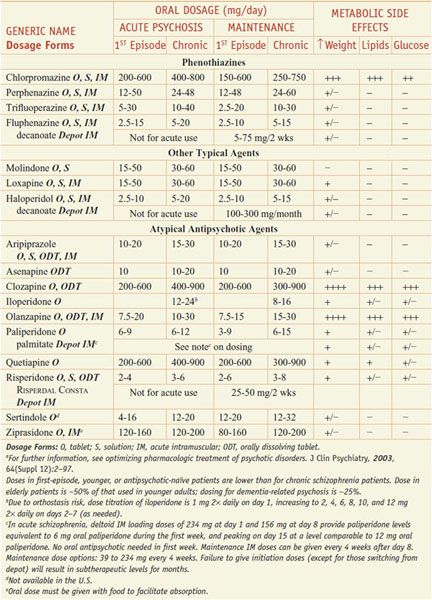
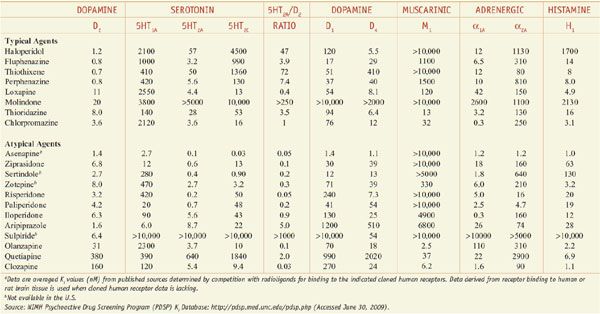
Acutely psychotic patients usually respond within hours after drug administration, but weeks may be required to achieve maximal drug response, especially for negative symptoms. Usual dosages for acute and maintenance treatment are noted in Table 16–1. Treatment-limiting adverse effects may include weight gain, sedation, orthostasis, and EPS, which to some degree can be predicted based on the potencies of the selected agent to inhibit neurotransmitter receptors (Table 16–2). The detection of dyslipidemia or hyperglycemia is based on laboratory monitoring (see Table 16–1). Certain adverse effects such as hyperprolactinemia, EPS, orthostasis, and sedation may respond to dose reduction, but metabolic abnormalities improve only with discontinuation of the drug and switching to a more metabolically benign medication. Patients with refractory schizophrenia on clozapine are not good candidates for switching because they are resistant to other medications (see the definition of refractory schizophrenia below).
Table 16–1
Drugs for Psychosis and Schizophrenia: Dosing and Metabolic Risk Profilea
Table 16–2
Potencies of Antipsychotic Agents at Neurotransmitter Receptorsa
The common problem of medication nonadherence among schizophrenia patients has led to the development of long-acting injectable (LAI) antipsychotic medications, often referred to as depot antipsychotics. There are currently 4 available LAI forms in the U.S.: decanoate esters of fluphenazine and haloperidol, risperidone-impregnated microspheres, and paliperidone palmitate. Patients receiving LAI antipsychotic medications show consistently lower relapse rates compared to patients receiving comparable oral forms and may suffer fewer adverse effects.
Lack of response to adequate antipsychotic drug doses for adequate periods of time may indicate treatment-refractory illness. Refractory schizophrenia is defined using the Kane criteria: failed 6-week trials of 2 separate agents and a third trial of a high-dose typical antipsychotic agent (e.g., haloperidol or fluphenazine 20 mg/day). In this patient population, response rates to typical antipsychotic agents, defined as 20% symptom reduction using standard rating scales (e.g., Positive and Negative Syndrome Scale [PANSS]), are 0%, and for any atypical antipsychotic except clozapine, are <10%. The therapeutic clozapine dose for a specific patient is not predictable, but various studies have found correlations between trough serum clozapine levels >327-504 ng/mL and likelihood of clinical response. When therapeutic serum concentrations are reached, response to clozapine occurs within 8 weeks.
Clozapine has numerous other adverse effects. These include agranulocytosis risk that mandates routine ongoing hematological monitoring, high metabolic risk, dose-dependent lowering of the seizure threshold, orthostasis, sedation, anticholinergic effects (especially constipation), and sialorrhea related to muscarinic agonism at M4 receptors. As a result, clozapine use is limited to refractory schizophrenia patients. Electroconvulsive therapy is considered a treatment of last resort in refractory schizophrenia and is rarely employed.
PHARMACOLOGY OF ANTIPSYCHOTIC AGENTS
Tables 16–1 and 16–2 summarize the drug classes, dose ranges, severity metabolic side effects, and potencies at important CNS receptors for a variety of antipsychotic agents.
The introduction of clozapine stimulated research into agents with antipsychotic activity and low EPS risk. This search led to a series of atypical antipsychotic agents with certain pharmacological similarities to clozapine: namely lower affinity for D2 receptors than typical antipsychotic drugs and high 5HT2 antagonist effects. Currently available atypical antipsychotic medications include the structurally related olanzapine, quetiapine, and clozapine; risperidone, its active metabolite paliperidone, and iloperidone; ziprasidone; lurasidone; asenapine, and aripiprazole (see Table 16–1).
MECHANISM OF ACTION. All clinically available antipsychotics are antagonists at dopamine D2 receptors. This reduction in dopaminergic neurotransmission is achieved through D2 antagonism or partial D2 agonism (e.g., aripiprazole).
Aripiprazole has an affinity for D2 receptors only slightly less than DA itself, but its intrinsic activity is ~25% that of DA. That is, when DA is incubated with increasing concentrations of aripiprazole, maximal inhibition of D2 activity do not exceed 25% of the DA response, the level of agonism provided by aripiprazole. Aripiprazole’s capacity to stimulate D2 receptors in brain areas where synaptic DA levels are limited (e.g., PFC neurons) or decrease dopaminergic activity when DA concentrations are high (e.g., mesolimbic cortex) is thought to be the basis for its clinical effects in schizophrenia. Even with 100% receptor occupancy, aripiprazole’s intrinsic dopaminergic agonism can generate a 25% postsynaptic signal, implying a maximal 75% reduction in DA neurotransmission, below the 78% threshold that triggers EPS in most individuals.
The pharmacological basis for the clinical efficacy atypical antipsychotics without EPS induction results from a significantly weaker D2 antagonism, combined with potent 5HT2 antagonism. Clozapine possesses activity at other receptors including antagonism and agonism at various muscarinic receptor subtypes and antagonism at dopamine D4 receptors. However D4 antagonists that do not have D2 antagonism lack antipsychotic activity. Clozapine’s active metabolite, N-desmethylclozapine, is a potent muscarinic M1 agonist.
The glutamate hypofunction hypothesis of schizophrenia has led to novel animal models that examine the influence of antipsychotic agents with agonist properties at metabotropic glutamate receptors mGlu2 and mGlu3 and other subtypes. Atypical antipsychotic drugs are better than typical antipsychotic medications at reversing the negative symptoms, cognitive deficits and social withdrawal induced by glutamate antagonists.
DOPAMINE RECEPTOR OCCUPANCY AND BEHAVIORAL EFFECTS. Excessive dopaminergic functions in the limbic system are central to the positive symptoms of psychosis. The behavioral effects and the time course of antipsychotic response parallel the rise in D2 occupancy and include calming of psychomotor agitation, decreased hostility, decreased social isolation, and less interference from disorganized or delusional thought processes and hallucinations. Occupation of ~78% of D2 receptors in the basal ganglia is associated with a risk of EPS across all DA antagonist antipsychotic agents, while occupancies in the range of 60-75% are associated with antipsychotic efficacy (see Figure 16–2). With the exception of aripiprazole, all atypical antipsychotic drugs at low doses have much greater occupancy of 5HT2A receptors (e.g., 75-99%) than typical agents (see Table 16–2).
D3 and D4 Receptors in the Basal Ganglia and Limbic System. D3 and D4 receptors are preferentially expressed in limbic areas. The D4 receptors, which are preferentially localized in cortical and limbic brain regions in low abundance, are upregulated after repeated administration of most typical and atypical antipsychotic drugs. These receptors may contribute to clinical antipsychotic actions, but agents that are D4 selective (e.g., sonepiprazole) or mixed D4/5HT2A antagonists (e.g., fananserin) lack antipsychotic efficacy in clinical studies.
D3 receptors are unlikely to play a pivotal role in antipsychotic drug actions. The subtle and atypical functional activities of cerebral D3 receptors suggest that D3 agonists rather than antagonists may have useful psychotropic effects, particularly in antagonizing stimulant-reward and dependence behaviors.
The Role of Non-Dopamine Receptors for Atypical Antipsychotic Agents. The concept of atypicality was initially based on clozapine’s absence of EPS within the therapeutic range, combined with a prominent role of 5HT2 receptor antagonism. As subsequent agents were synthesized using clozapine’s 5HT2/D2 ratio as a model, most of which possessed greater D2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree