Pharmacotherapy of Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a spectrum of chronic, idiopathic, inflammatory intestinal conditions. IBD causes significant gastrointestinal (GI) symptoms that include diarrhea, abdominal pain, bleeding, anemia, and weight loss. IBD conventionally is divided into 2 major subtypes: ulcerative colitis and Crohn disease. Ulcerative colitis is characterized by confluent mucosal inflammation of the colon starting at the anal verge and extending proximally for a variable extent (e.g., proctitis, left-sided colitis, or pancolitis). Crohn disease, by contrast, is characterized by transmural inflammation of any part of the GI tract but most commonly the area adjacent to the ileocecal valve. The inflammation in Crohn disease is not necessarily confluent, frequently leaving “skip areas” of relatively normal mucosa. The transmural nature of the inflammation may lead to fibrosis and strictures or fistula formation.
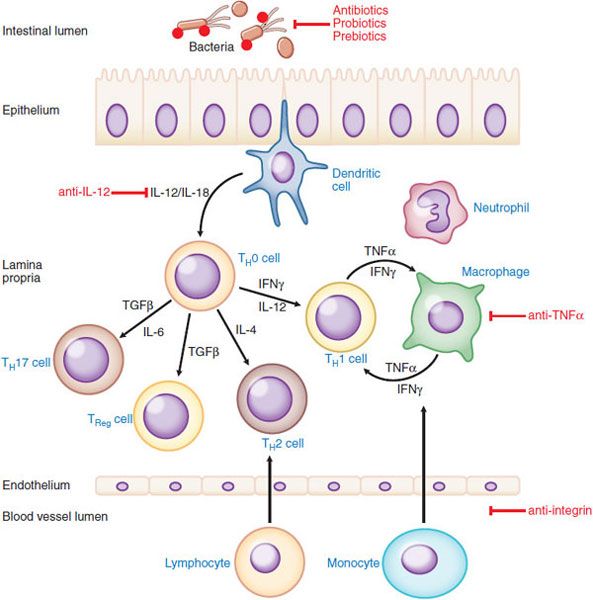
PATHOGENESIS OF IBD. Crohn disease and ulcerative colitis are chronic idiopathic inflammatory disorders of the GI tract; a summary of proposed pathogenic events and potential sites of therapeutic intervention is shown in Figure 47–1. Crohn disease and ulcerative colitis result from distinct pathogenetic mechanisms. Histologically, the transmural lesions in Crohn disease exhibit marked infiltration of lymphocytes and macrophages, granuloma formation, and submucosal fibrosis, whereas the superficial lesions in ulcerative colitis have lymphocytic and neutrophilic infiltrates. Within the diseased bowel in Crohn disease, the cytokine profile includes increased levels of interleukin (IL)-12, IL-23, interferon-γ, and tumor necrosis factor-α (TNFα), findings characteristic of T-helper 1 (TH1)–mediated inflammatory processes. In contrast, the inflammatory response in ulcerative colitis resembles aspects of that mediated by the TH2 pathway. Understanding of the inflammatory processes has evolved with the description of regulatory T cells and pro-inflammatory TH17 cells, a novel T-cell population that expresses IL-23 receptor as a surface marker and produces, among others, the pro-inflammatory cytokines IL-17, IL-21, IL-22, and IL-26. TH17 cells seem to play a prominent role in intestinal inflammation, particularly in Crohn disease.
Figure 47–1 Proposed pathogenesis of inflammatory bowel disease and target sites for pharmacological intervention. Shown are the interactions among bacterial antigens in the intestinal lumen and immune cells in the intestinal wall. If the epithelial barrier is impaired, bacterial antigens can gain access to antigen-presenting cells (APC) such as dendritic cells in the lamina propria. These cells then present the antigen(s) to CD4+ lymphocytes and also secrete cytokines such interleukin (IL)-12 and IL-18, thereby inducing the differentiation of TH1 cells in Crohn’s disease (or, under the control of IL-4, type 2 helper T cells [TH2] in ulcerative colitis). The balance of pro-inflammatory and anti-inflammatory events is also governed by regulatory TH17 and TReg cells, both of which serve to limit immune and inflammatory responses in the GI tract. Transforming growth factor (TGF)β and IL-6 are important cytokines that drive the expansion of the regulatory T cell subsets. The TH1 cells produce a characteristic array of cytokines, including interferon (IFN) γ and TNFα, which in turn activate macrophages. Macrophages positively regulate TH1 cells by secreting additional cytokines, including IFNγ and TNFα. Recruitment of a variety of leukocytes is mediated by activation of resident immune cells including neutrophils. Cell adhesion molecules such as integrins are important in the infiltration of leukocytes and novel biological therapeutic strategies aimed at blocking leukocyte recruitment are effective at reducing inflammation. General immunosuppressants (e.g., glucocorticoids, thioguanine derivatives, methotrexate, and cyclosporine) affect multiple sites of inflammation. More site-specific intervention involve intestinal bacteria (antibiotics, prebiotics, and probiotics) and therapy directed at TNFα or IL-12.
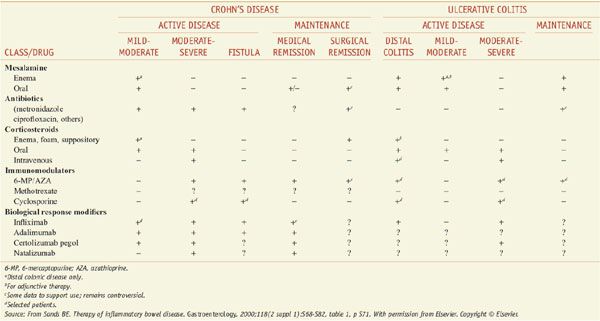
PHARMACOTHERAPY FOR IBD. Medical therapy for IBD is problematic. Because no unique abnormality has been identified, therapy for IBD seeks to dampen the generalized inflammatory response. Regrettably, no agent can reliably accomplish this, and the response of an individual patient to a given medicine may be limited and unpredictable. Specific goals of pharmacotherapy in IBD include controlling acute exacerbations of the disease, maintaining remission, and treating specific complications such as fistulas. The major therapeutic options are considered below and summarized at chapter’s end by Table 47–1.
Table 47–1
Medications Commonly Used to Treat Inflammatory Bowel Disease
MESALAMINE (5-ASA)-BASED THERAPY
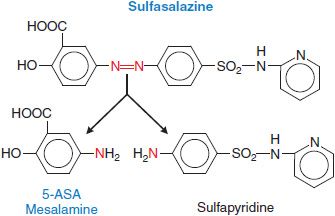
First-line therapy for mild to moderate ulcerative colitis generally involves mesalamine (5-aminosalicylic acid, or 5-ASA). The archetype for this class of medications is sulfasalazine (AZULFIDINE), which consists of 5-ASA linked to sulfapyridine by an azo bond (Figure 47–2).
Figure 47–2 Generation of mesalamine from the prodrug sulfasalazine. The red N atoms indicate the diazo linkage that is cleaved in the colon to generate the active moiety.
MECHANISM OF ACTION AND PHARMACOLOGICAL PROPERTIES. Sulfasalazine is an oral prodrug that effectively delivers 5-ASA to the distal GI tract. The azo linkage in sulfasalazine prevents absorption in the stomach and small intestine, and the individual components are not liberated for absorption until colonic bacteria cleave the bond. 5-ASA is the therapeutic moiety, with little, if any, contribution by sulfapyridine. Although 5-ASA is a salicylate, its therapeutic effect does not appear to be related to cyclooxygenase inhibition; indeed, traditional nonsteroidal anti-inflammatory drugs may exacerbate IBD. Many potential sites of action (effects on immune function and inflammation) have been demonstrated in vitro for either sulfasalazine or mesalamine (inhibition of the production of IL-1 and TNFα, inhibition of the lipoxygenase pathway, scavenging of free radicals and oxidants, and inhibition of NFκB, a transcription factor pivotal to production of inflammatory mediators), however, specific mechanisms of action have not been identified.
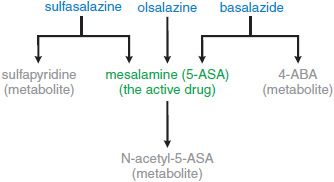
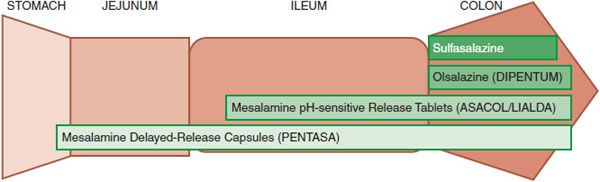
Although not active therapeutically, sulfapyridine causes many of the adverse effects observed in patients taking sulfasalazine. To preserve the therapeutic effect of 5-ASA without the adverse effects of sulfapyridine, several second-generation 5-ASA compounds have been developed (Figures 47–2, 47–3, and 47–4). They are divided into 2 groups: prodrugs and coated drugs. Prodrugs contain the same azo bond as sulfasalazine but replace the linked sulfapyridine with either another 5-ASA (olsalazine, DIPENTUM) or an inert compound (balsalazide, COLAZIDE). The alternative approaches employ either a delayed-release formulation (PENTASA) or a pH-sensitive coating (ASACOL; LIALDA/MEZAVANT). Delayed-release mesalamine is released throughout the small intestine and colon, whereas pH-sensitive mesalamine is released in the terminal ileum and colon. These different distributions of drug delivery have potential therapeutic implications.
Figure 47–3 Metabolic fates of the different oral formulations of mesalamine (5-ASA).
Figure 47–4 Sites of release of mesalamine (5-ASA) in the GI tract from different oral formulations.
Oral sulfasalazine is effective in patients with mild or moderately active ulcerative colitis, with response rates of 60-80%. The usual dose is 4 g/day in 4 divided doses with food; to avoid adverse effects, the dose is increased gradually from an initial dose of 500 mg twice a day. Doses as high as 6 g/day can be used but cause an increased incidence of side effects. For patients with severe colitis, sulfasalazine is of less certain value, even though it is often added as an adjunct to systemic glucocorticoids. The drug plays a useful role in preventing relapses once remission has been achieved. Because they lack the dose-related side effects of sulfapyridine, the newer formulations can be used to provide higher doses of mesalamine with some improvement in disease control. The usual doses to treat active disease are 800 mg 3 times a day for ASACOL and 1 g 4 times a day for PENTASA. Lower doses are used for maintenance (e.g., ASACOL, 800 mg twice a day). The efficacy of 5-ASA preparations (e.g., sulfasalazine) in Crohn disease is less striking, with modest benefit at best in controlled trials. The second-generation 5-ASA prodrugs (e.g., olsalazine and balsalazide) do not have a significant effect in small-bowel Crohn disease.
Topical preparations of mesalamine suspended in a wax matrix suppository (ROWASA) or in a suspension enema (CANASA) are effective in active proctitis and distal ulcerative colitis, respectively. They appear to be superior to topical hydrocortisone in this setting, with response rates of 75-90%. Mesalamine enemas (4 g/60 mL) should be used at bedtime and retained for at least 8 h; the suppository (500 mg) should be used 2 to 3 times a day with the objective of retaining it for at least 3 h. Response to local therapy with mesalamine may occur within 3-21 days; however, the usual course of therapy is from 3-6 weeks. Once remission has occurred, lower doses are used for maintenance.
ADME.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree