Muscarinic Receptor Agonists and Antagonists
ACETYLCHOLINE AND ITS MUSCARINIC RECEPTOR TARGET
Muscarinic acetylcholine receptors in the peripheral nervous system occur primarily on autonomic effector cells innervated by postganglionic parasympathetic nerves. Muscarinic receptors are also present in autonomic ganglia and on some cells (e.g., vascular endothelial cells) that, paradoxically, receive little or no cholinergic innervation. Within the CNS, the hippocampus, cortex, and thalamus have high densities of muscarinic receptors. Acetylcholine (ACh), the naturally occurring neurotransmitter for these receptors, has virtually no systemic therapeutic applications because its actions are diffuse, and its hydrolysis, catalyzed by both acetylcholinesterase (AChE) and plasma butyrylcholinesterase, is rapid. Muscarinic agonists mimic the effects of ACh at these sites and are longer-acting congeners of ACh or natural alkaloids.
Cholinergic synapses are found at:
• Autonomic effector sites innervated by postganglionic parasympathetic nerves (or, in the sweat glands, by postganglionic sympathetic nerves)
• Sympathetic and parasympathetic ganglia and the adrenal medulla, innervated by preganglionic autonomic nerves
• Motor end plates on skeletal muscle, innervated by somatic motor nerves
• Certain synapses in the CNS, where ACh can have either pre- or postsynaptic actions
The actions of ACh and related drugs at autonomic effector sites are termed muscarinic, based on the observation that the alkaloid muscarine acts selectively at those sites and produces the same qualitative effects as ACh (see Table 8–1). Muscarinic receptors are present in autonomic ganglia and the adrenal medulla but primarily function to modulate the nicotinic actions of ACh at these sites (see Chapter 11). In the CNS, muscarinic receptors are widely distributed and mediate many important responses. The actions of ACh and its congeners at muscarinic receptors can be blocked competitively by atropine.
PROPERTIES AND SUBTYPES OF MUSCARINIC RECEPTORS
Muscarinic receptors comprise 5 distinct gene products, designated as M1 through M5 (see Table 8–3). Muscarinic receptors are GPCRs that in turn couple to various cellular effectors. Selectivity is not absolute but, in general, stimulation of M1, M3, and M5 receptors activates the Gq-PLC-IP3/DAG-Ca2+ pathway, resulting in a variety of Ca2+-mediated responses. By contrast, M2 and M4 muscarinic receptors couple to the pertussis toxin–sensitive G proteins, Gi and Go, to inhibit adenylyl cyclase and regulate specific ion channels.
The 5 muscarinic receptor subtypes are widely distributed in both the CNS and peripheral tissues; most cells express at least 2 subtypes (see Table 8–3). The M2 receptor is the predominant subtype in the cholinergic control of the heart, whereas the M3 receptor is the predominant subtype in the cholinergic control of smooth muscle, secretory glands, and the eye. The M1 receptor has an important role in the modulation of nicotinic cholinergic transmission in ganglia.
PHARMACOLOGICAL EFFECTS OF ACETYLCHOLINE
CARDIOVASCULAR SYSTEM. ACh has 4 primary effects on the cardiovascular system:
• Vasodilation
• Decrease heart rate (negative chronotropic effect)
• Decrease the conduction velocity in the atrioventricular (AV) node (negative dromotropic effect)
• Decrease in the force of cardiac contraction (negative inotropic effect)
The negative inotropic effect is less significant in the ventricles than in the atria. Some of these responses can be obscured by baroreceptor and other reflexes that dampen the direct responses to ACh. Although ACh rarely is given systemically, its cardiac actions are important because the cardiac effects of cardiac glycosides, anti-arrhythmic agents, and many other drugs are at least partly due to changes in parasympathetic (vagal) stimulation of the heart; in addition, afferent stimulation of the viscera during surgical interventions can reflexly increase the vagal stimulation of the heart.
The intravenous injection of a small dose of ACh produces a transient fall in blood pressure owing to generalized vasodilation (mediated by vascular endothelial NO), which is usually accompanied by reflex tachycardia. A considerably larger dose is required to see direct effects of ACh on the heart, such as eliciting bradycardia or AV nodal conduction block. The generalized vasodilation produced by exogenously administered ACh is due to the stimulation of muscarinic receptors, primarily of the M3 subtype, located on vascular endothelial cells despite the apparent lack of cholinergic innervation. Occupation of the receptors by agonist activates the Gq-PLC-IP3 pathway, leading to Ca2+-calmodulin–dependent activation of endothelial NO synthase and production of NO, which diffuses to adjacent vascular smooth muscle cells and causes them to relax (see Chapters 3 and 8). If the endothelium is damaged, as occurs under various pathophysiological conditions, ACh acts predominantly on M3 receptors located on vascular smooth muscle cells, causing vasoconstriction.
ACh affects cardiac function directly and also indirectly through inhibition of the adrenergic stimulation of the heart. Cardiac effects of ACh are mediated primarily by M2 muscarinic receptors, which couple to Gi/Go. The direct effects include:
• Increase in the ACh-activated K+ current (IK-ACh) due to activation of K-ACh channels
• Decrease in the L-type Ca2+ current (ICa-L) due to inhibition of L-type Ca2+ channels
• Decrease in the cardiac pacemaker current (If) due to inhibition of HCN (pacemaker) channels
The indirect effects include:
• Gi-mediated decrease in cyclic AMP, which opposes and counteracts the β1 receptor/Gs–mediated increase in cyclic AMP
• Inhibition of the release of NE from sympathetic nerve terminals
The inhibition of NE release is mediated by presynaptic M2 and M3 receptors, which are stimulated by ACh released from adjacent parasympathetic postganglionic nerve terminals. Presynaptic M2 receptors also inhibit ACh release from parasympathetic postganglionic nerve terminals in the human heart.
ACh slows the heart rate primarily by decreasing the rate of spontaneous depolarization of the SA node (see Chapter 29); attainment of the threshold potential and the succeeding events in the cardiac cycle are therefore delayed. In the atria, ACh causes hyperpolarization and a decreased action potential duration by increasing IK-ACh. ACh also inhibits cyclic AMP formation and NE release, decreasing atrial contractility. The rate of impulse conduction is either unaffected or may increase in response to ACh; the increase probably is due to the activation of additional Na+ channels in response to ACh-induced hyperpolarization. In contrast, in the AV node (which has Ca2+ channel-dependent action potentials; see Chapter 29), ACh slows conduction and increases the refractory period by inhibiting ICa-L; the decrement in AV conduction is responsible for the complete heart block that may be observed when large quantities of cholinergic agonists are administered systemically.
Cholinergic (vagal) innervation of the His-Purkinje system and ventricular myocardium is sparse and the effects of ACh are smaller than those observed in the atria and nodal tissues. In the ventricles, ACh, whether released by vagal stimulation or applied directly, has a small negative inotropic effect; this inhibition is most apparent when there is concomitant adrenergic stimulation or underlying sympathetic tone. Automaticity of Purkinje fibers is suppressed, and the threshold for ventricular fibrillation is increased.
RESPIRATORY TRACT. The parasympathetic nervous system plays a major role in regulating bronchomotor tone. The effects of ACh on the respiratory system include not only bronchoconstriction but also increased tracheobronchial secretion and stimulation of the chemoreceptors of the carotid and aortic bodies. These effects are mediated primarily by M3 muscarinic receptors.
URINARY TRACT. Parasympathetic sacral innervation causes detrusor muscle contraction, increased voiding pressure, and ureteral peristalsis. M2 receptors appear most prevalent in the bladder; M3 receptor mediates detrusor muscle contraction.
GI TRACT. Stimulation of vagal input to the GI tract increases tone, amplitude of contractions, and secretory activity of the stomach and intestine. The muscarinic receptors of the M2 subtype are most prevalent, but M3 muscarinic receptors are primarily responsible for mediating the cholinergic control of GI motility.
SECRETORY AND OCULAR EFFECTS. ACh stimulates secretion from glands that receive parasympathetic or sympathetic cholinergic innervation, including the lacrimal, nasopharyngeal, salivary, and sweat glands. These effects are mediated primarily by M3 muscarinic receptors; M1 receptors also contribute significantly to the cholinergic stimulation of salivary secretion. When instilled into the eye, ACh produces miosis by contracting the pupillary sphincter muscle and accommodation for near vision by contracting the ciliary muscle (see Chapter 64); both effects are mediated primarily by M3 muscarinic receptors.
CNS EFFECTS. All 5 muscarinic receptor subtypes are found in the brain, and studies suggest that muscarinic receptor-regulated pathways may have an important role in cognitive function, motor control, appetite regulation, nociception, and other processes. Elevation of ACh with AChE inhibitors is used in treating some of the cognitive symptoms of Alzheimer disease (see Table 22–2).
MUSCARINIC RECEPTOR AGONISTS
Muscarinic cholinergic receptor agonists can be divided into 2 groups:
• Choline esters, including ACh and several synthetic esters
• Naturally occurring cholinomimetic alkaloids (particularly pilocarpine, muscarine, and arecoline) and their synthetic congeners
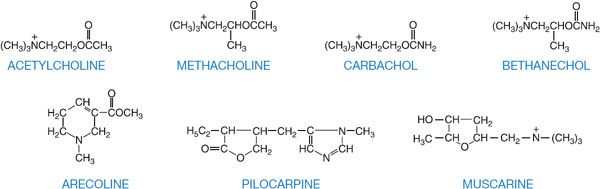
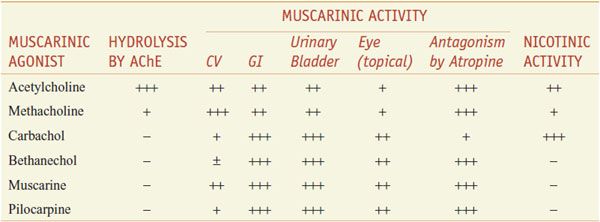
Of several hundred synthetic choline derivatives investigated, only methacholine, carbachol, and bethanechol have had clinical applications, along with a few natural alkaloids (Figure 9–1; Table 9–1).
Figure 9–1 Structural formulas of acetylcholine, choline esters, and natural alkaloids that stimulate muscarinic receptors.
Table 9–1
Some Pharmacological Properties of Choline Esters and Natural Alkaloids
Methacholine (acetyl-β-methylcholine), the β-methyl analog of ACh, is a synthetic choline ester that differs from ACh chiefly in its greater duration of action (the added methyl group increases its resistance to hydrolysis by cholinesterases) and its predominantly muscarinic selectivity. Carbachol and bethanechol are unsubstituted carbamoyl esters that are completely resistant to hydrolysis by cholinesterases; their t1/2 are thus sufficiently long that they become distributed to areas of low blood flow. Carbachol retains substantial nicotinic activity, particularly on autonomic ganglia. Bethanechol has mainly muscarinic actions, with prominent effects on motility of the GI tract and urinary bladder. The major natural alkaloid muscarinic agonists are muscarine, pilocarpine, and arecoline. Muscarine acts almost exclusively at muscarinic receptor sites and is of toxicological significance (see below). Pilocarpine has a dominant muscarinic action but is a partial rather than a full agonist; the sweat glands are particularly sensitive to pilocarpine. Arecoline acts at nicotinic receptors. Although these naturally occurring alkaloids are of great value as pharmacological tools, present clinical use is restricted largely to the employment of pilocarpine as a sialagogue and miotic agent (see Chapter 64).
ABSORPTION, DISTRIBUTION, AND ELIMINATION. Muscarine and the choline esters are quaternary amines (see Figure 9–1), hence they are poorly absorbed following oral administration and have a limited ability to cross the blood-brain barrier. The choline esters are short-acting agents due to rapid elimination by the kidneys. Muscarine can still, however, be toxic when ingested and can even have CNS effects. Pilocarpine and arecoline, being tertiary amines, are readily absorbed and can cross the blood-brain barrier. Pilocarpine clearance is decreased in patients with hepatic impairment. The natural alkaloids are primarily eliminated by the kidneys; excretion of the tertiary amines can be accelerated by acidification of the urine.
THERAPEUTIC USES OF MUSCARINIC RECEPTOR AGONISTS
Muscarinic agonists are currently used in the treatment of urinary bladder disorders and xerostomia and in the diagnosis of bronchial hyper-reactivity. They are also used in ophthalmology as miotic agents and for the treatment of glaucoma. There is growing interest in the role of muscarinic receptors in cognition and the potential utility of M1 agonists in treating the cognitive impairment associated with Alzheimer disease.
ACETYLCHOLINE. ACh (MIOCHOL-E) is used topically for the induction of miosis during ophthalmologic surgery; it is instilled into the eye as a 1% solution (see Chapter 64).
METHACHOLINE. Methacholine (PROVOCHOLINE) is administered by inhalation for the diagnosis of bronchial airway hyperreactivity in patients who do not have clinically apparent asthma. Contraindications to methacholine testing include severe airflow limitation, recent myocardial infarction or stroke, uncontrolled hypertension, or pregnancy. The response to methacholine also may be exaggerated or prolonged in patients taking β adrenergic receptor antagonists. Methacholine is available as a powder that is diluted with 0.9% NaCl and administered via a nebulizer.
BETHANECHOL. Bethanechol (URECHOLINE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree