Contraception and Pharmacotherapy of Obstetrical and Gynecological Disorders
Drugs to control fertility and treat disorders of the female reproductive organs collectively are among the most frequently prescribed agents in clinical practice. This chapter discusses a number of common clinical issues and their drug therapies that are central to women’s health. The focus is on reproductive disorders and aspects of therapy rather than comprehensive coverage of the drugs themselves, which are described in more detail elsewhere (e.g., see Chapter 33 for prostaglandins; Chapter 38 for the gonadotropins, gonadotropin-releasing hormone [GnRH] agonists and antagonists, and oxytocin; Chapter 40 for estrogens and progestins; Section VII for antibiotics).
CONTRACEPTION
Contraception can be administered as planned prophylaxis or postcoitally for emergency contraception (i.e., high-dose estrogen-containing oral contraceptive pills, high-dose progestin pills, a progesterone antagonist, intrauterine devices). A progesterone antagonist also can be used to terminate an established pregnancy.
PLANNED CONTRACEPTION
COMBINATION ORAL CONTRACEPTIVES. Pills containing an estrogen and progestin are the most widely used (Table 66–1); they act primarily by suppressing the luteinizing hormone (LH) surge and thereby preventing ovulation. A wide variety of preparations are available for oral, transdermal, and vaginal administration (see Table 66–2 in the 12th edition of the parent text for a list of branded formulations, many of which are available as generics). Almost all contain ethinyl estradiol as the estrogen and a 17α-alkyl-19-nortestosterone derivative as the progestin, and are administered for the first 21-24 days of a 28-day cycle.
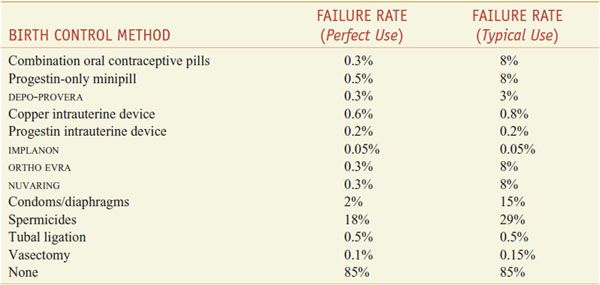
Table 66–1
One-Year Failure Rate with Various Forms of Contraception
Table 66–2
Sexually Transmitted Gynecological Infections and Recommended Therapie
MECHANISMS OF ACTION. Estrogen sensitizes the hypothalamus and pituitary gonadotropes to the feedback inhibitory effects of the progestin and minimizes breakthrough bleeding. The progestin exerts negative feedback, which suppresses the LH surge and thereby prevents ovulation, and protects against uterine cancer by opposing the proliferative effects of the estrogen on the uterine endometrium.
FORMULATIONS. Newer formulations offer effective contraception with improved activity profiles. They contain lower amounts of hormones to minimize adverse effects; some incorporate progestins with less androgenic activity (e.g., gestodene, desogestrel) or that antagonize the mineralocorticoid receptor and thereby reduce the tendency toward edema (e.g., drospirenone). Traditionally, combination oral contraceptives were packaged with 21 pills containing active hormone and 7 placebo tablets; each active pill contained a constant amount of the estrogen and progestin (i.e., a monophasic formulation). In an effort to maximize the antiovulatory effects and prevent breakthrough bleeding while minimizing total exposure to the hormones, some formulations provide active pills with 2 (biphasic) or 3 (triphasic) different amounts of 1 or both hormones to be used sequentially during each cycle.
“Extended-cycle” contraceptives extend the number of active pills per cycle and thus decrease the duration of menstrual bleeding. Two products contain 24 active pills with only 4 placebo tablets (e.g., YAZ [which contains drospirenone as the progestin] and LOESTRIN 24). Two products are packaged as 91-day packets, with 84 estrogen/progestin tablets and 7 placebo tablets (SEASONALE) or 7 tablets containing a lower dose of ethinyl estradiol alone (SEASONIQUE). Finally, LYBREL is provided in 28-day packets that contain only hormone pills and no placebo. All of these extended-cycle formulations appear to be comparable to the traditional products as contraceptives; aside from an increased frequency of breakthrough bleeding initially, no unexpected adverse effects have been observed.
A weekly transdermal contraceptive patch (ORTHO EVRA) releases ethinyl estradiol (20 μg/day) and norelgestromin (which is metabolized to norgestimate; 150 μg/day). In response to pharmacokinetic data suggesting that this patch provides higher estrogen exposure (AUC) than the low-dose oral contraceptive pills, the FDA added a black box advisory that notes this pharmacokinetic difference and warns of a potential increased risk of venous thromboembolism. Local reactions to the patch occur in ~5-15% of users and may be decreased by pre-application of a topical glucocorticoid. A vaginal ring (NUVARING) also is available that releases ethinyl estradiol (15 μg daily) and etonogestrel (an active metabolite of desogestrel; 120 μg daily). Each ring is used for 3 weeks, followed by a 1-week interval without the ring.
The combination estrogen/progestin formulations provide highly effective (~99%) contraception and also have a number of noncontraceptive benefits, including protection against certain cancers (e.g., ovarian, endometrial, colorectal), decreased iron-deficiency anemia secondary to menstrual blood loss, and decreased risk of fractures due to osteoporosis. Combination oral contraceptives also are widely used for conditions such as endometriosis, dysmenorrhea, menorrhagia, irregular menstrual cycles, premenstrual dysphoric disorder, acne, and hirsutism.
ADVERSE EFFECTS. Serious adverse effects of the combination estrogen/progestin contraceptive agents are relatively rare. Thromboembolic disease, largely due to the estrogenic component, is the most common serious side effect. Estrogen concentration, the patient’s age, smoking, and inherited thrombophilias all influence the risk of developing thromboembolic disease. The impact of combination oral contraceptives on breast cancer has been highly debated; although a meta-analysis of epidemiological studies concluded that the combination oral contraceptives did increase the risk of breast cancer, studies conducted with the lower doses of hormones in current formulations suggest that the risk of breast cancer is not increased.
Other adverse effects include hypertension, edema, gallbladder disease, and elevations in serum triglycerides (see Chapter 40). With pills containing drospirenone, which antagonizes the mineralocorticoid receptor, serum K+ should be monitored in women at risk for hyperkalemia (e.g., those on K+-sparing diuretics or drugs that inhibit the renin–angiotensin system). The combination oral contraceptives are contraindicated in women with a history of thromboembolic disease, cerebrovascular disease, migraine headaches with aura, estrogen-dependent cancer, impaired hepatic function or active liver disease, undiagnosed uterine bleeding, and suspected pregnancy. Patients with a history of gestational diabetes should be monitored closely, and drug cessation should be strongly considered in anticipation of events associated with an increased risk of venous thromboembolism (e.g., elective surgery).
PROGESTIN-ONLY CONTRACEPTIVES. Progestin-only minipills contain derivatives of 17α-alkyl-19-nortestosterone but no estrogen. Although they do inhibit ovulation to some degree, their efficacy also reflects changes in the cervical mucus that inhibit fertilization and endometrial changes that inhibit implantation. They are slightly less effective than the combination estrogen/progestin formulations. Their major adverse effect is breakthrough bleeding.
Progestins also are used for long-acting contraception. A depot formulation of medroxyprogesterone (DEPO-PROVERA) injected subcutaneously or intramuscularly provides effective contraception for 3 months. Its use has been associated with decreased bone mineral density. Subdermal implants of progestin-impregnated rods provide effective contraception over several years. The only implant system currently approved in the U.S. is IMPLANON, which incorporates 3-ketodesogestrel, an active metabolite of desogestrel, into an inert matrix. An intrauterine device that releases levonorgestrel (MIRENA) provides highly effective contraception for up to 5 years. It acts predominantly to inhibit gamete function and survival via local changes in the cervical mucus.
POSTCOITAL CONTRACEPTION
Postcoital (or emergency) contraception is indicated for use in cases of mechanical failure of barrier devices or in circumstances of unprotected intercourse. It is not intended as a regular method of contraception. The mechanisms of action of the postcoital contraceptives are not fully understood.
PLAN-B one-step, which contains 2 tablets of the progestin levonorgestrel (0.75 mg each), is marketed specifically for postcoital contraception and may be obtained in the U.S. without a prescription by women >18 years of age. Treatment is most effective if the first dose is taken within 72 h of intercourse, followed by a second dose 12 h later; a single dose of 1.5 mg within 72 h of intercourse appears to be equally effective. Other options for postcoital contraception include mifepristone (MIFEPREX), which is not FDA-approved for this indication but is highly effective in oral doses ranging from 10-50 mg when taken within 5 days after unprotected intercourse, and copper intrauterine devices when inserted within 4 days of unprotected intercourse. Mifepristone also has abortifacient activity when used in a different treatment regimen. The selective progesterone receptor modulator ulipristal (ELLA, ELLAONE) was recently approved as an emergency contraceptive, effective up to 120 h after unprotected intercourse; see Chapter 40 for details.
PREGNANCY TERMINATION
If contraception is not used or fails, either mifepristone (RU-486, MIFEPREX) or methotrexate (50 mg/m2 intramuscularly or orally) can be used to terminate an unwanted pregnancy in settings outside surgical centers. A prostaglandin then is administered to stimulate uterine contractions and expel the detached conceptus; in the U.S., prostaglandins used include dinoprostone (PGE2; PROSTIN E2) administered vaginally or the PGE1 analog misoprostol (CYTOTEC) given orally or vaginally, both of which are used off label for this purpose. Prostaglandins used in other countries include the PGE2 analog sulprostone (NALADOR) and the PGE1 analog gemeprost (CERVAGEM).
MIFEPRISTONE. Mifepristone is a 17α-alkyl-19-nortestosterone derivative that acts as a competitive antagonist at the progesterone receptor. Its actions are associated with focal hemorrhage and breakdown of the stromal extracellular matrix that ultimately leads to the breakdown of the uterine endometrium. In addition, mifepristone increases the sensitivity of the uterus to the uterotonic effects of prostaglandins. Mifepristone is metabolized through a series of reactions initiated by hepatic CYP3A4. Women receiving chronic glucocorticoid therapy should not be given mifepristone because of its anti-glucocorticoid activity, and the drug should be used cautiously in women who are anemic or receiving anticoagulants.
As approved by the FDA, mifepristone (600 mg) is taken for pregnancy termination within 49 days after the start of a woman’s last menstrual period. The synthetic PGE1 analog misoprostol (400 μg) is administered orally 48 h later; vaginal administration is at least as effective but is not FDA-approved. Complete abortion using this procedure exceeds 90%; when termination of pregnancy fails or is incomplete, surgical intervention is required. Repeated doses of misoprostol alone (e.g., 800 μg vaginally or sublingually every 3 h or every 12 h for 3 doses) also have been effective in settings where mifepristone is unavailable. Vaginal bleeding follows pregnancy termination and typically lasts from 1-2 weeks but rarely (in 0.1% of patients) is severe enough to require blood transfusion. A high percentage of women also experience abdominal pain and uterine cramps, nausea and vomiting, and diarrhea secondary to the prostaglandin. Because mifepristone carries a risk of serious, and sometimes fatal, infection and bleeding following its use for medical abortion, a black box warning has been added to the product labeling. Women receiving mifepristone should be informed of these risks and cautioned to seek immediate medical attention if symptoms or signs of these conditions occur. Fulminant septic shock associated with Clostridium sordellii infections may result and is attributable to the combined effects of uterine infection and inhibition of glucocorticoid action by mifepristone.
METHOTREXATE. Methotrexate is a potent abortifacient, probably as a result of the ability of the placenta to concentrate FH2Glun (dihydrofolate polyglutamate) and its analogs (see Chapter 61).
DRUG THERAPY IN GYNECOLOGY
INDUCTION OF SEXUAL MATURATION
A number of clinical disorders, including Turner syndrome and other forms of gonadal dysgenesis, are associated with impaired production of ovarian steroids in phenotypic females. Such patients typically fail to develop secondary sexual characteristics at the normal time of puberty (sexual infantilism) or fail to have menses (primary amenorrhea). In these cases, steroid hormones are administered to induce development of the secondary sex characteristics; however, treatment is initiated only after the diagnosis is ascertained and underlying disorders that might respond to more specific therapy (e.g., prolactinomas) are excluded (see Chapter 38).
Types of estrogens used and the treatment regimens may vary by country or individual preference. Examples include conjugated estrogens, 0.3-1.25 mg; micronized 17β-estradiol, 0.5-2.0 mg; ethinyl estradiol, 5-20 μg; and transdermal 17β-estradiol, 25-50 μg. To achieve optimal breast development, treatment typically is initiated with a low dose of estrogen (e.g., conjugated estrogens at a starting dosage of 0.3 mg/day or ethinyl estradiol at 5 μg/day) starting in patients between ages 10 and 12 years or immediately if the diagnosis is made after this age. After 3-6 months, the dosage is increased (e.g., 0.9-1.25 mg/day of conjugated estrogens or 20 μg/day of ethinyl estradiol). Once this is achieved, a progestin (e.g., medroxyprogesterone, 10 mg/day, or micronized progesterone, 200-400 μg/day) for 12 days each cycle is added to the regimen to optimize breast development and permit cyclical menses, thereby avoiding endometrial hyperplasia and its consequent risk of uterine cancer. Once menses are established, many clinicians will switch to a standard low-dose oral contraceptive pill or even may use an extended-cycle formulation.
Short stature, a universal feature of non-mosaic Turner syndrome, usually is treated with human growth hormone, often together with an androgen such as oxandrolone (see Chapter 41). Initiating treatment with human growth hormone and androgen and delaying the onset of estrogen therapy generally produces a better growth response (see Chapter 38).
MENOPAUSE
Menopause refers to the permanent cessation of menstrual periods (i.e., for >12 months) resulting from the loss of ovarian follicular activity; it usually occurs when women are between 45 and 60 years of age.
The decline in estradiol levels produces a variety of symptoms and signs, including vasomotor disturbances (hot flashes or flushes), sweating, irritability, sleep disturbances, and atrophy of estrogen-dependent tissue. In addition, postmenopausal women are at increased risk for osteoporosis, bone fractures, and coronary heart disease and experience increased memory loss and other cognitive difficulties.
ESTROGEN THERAPY. The observed estrogen deficiency associated with menopause, as well as a number of studies showing positive effects of estrogen replacement therapy on these parameters, led to widespread use of hormone replacement therapy in peri- and postmenopausal women.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree