Pharmacogenomics of Gastrointestinal Drugs: Focus on Proton Pump Inhibitors
LEARNING OBJECTIVES
INTRODUCTION
Proton pump inhibitors (PPIs), such as omeprazole, lansoprazole, rabeprazole, esomeprazole, and pantoprazole, are now clinically used as the potent gastric acid inhibitors. They are derivatives of benzimidazole. They are absorbed in the small intestine and reach, via systemic circulation, the gastric parietal cells, where they bind to the proton pump (H+/K+-ATPase) irreversibly and disturb the function of proton pump, thereby resulting in a potent acid inhibition.1 The major indications of PPIs are acid-related diseases, such as peptic ulcer, gastroesophageal reflux diseases (GERD), and Zollinger–Ellison syndrome.2–6 PPIs are also used for the eradication of H. pylori with antimicrobial agents.7–9
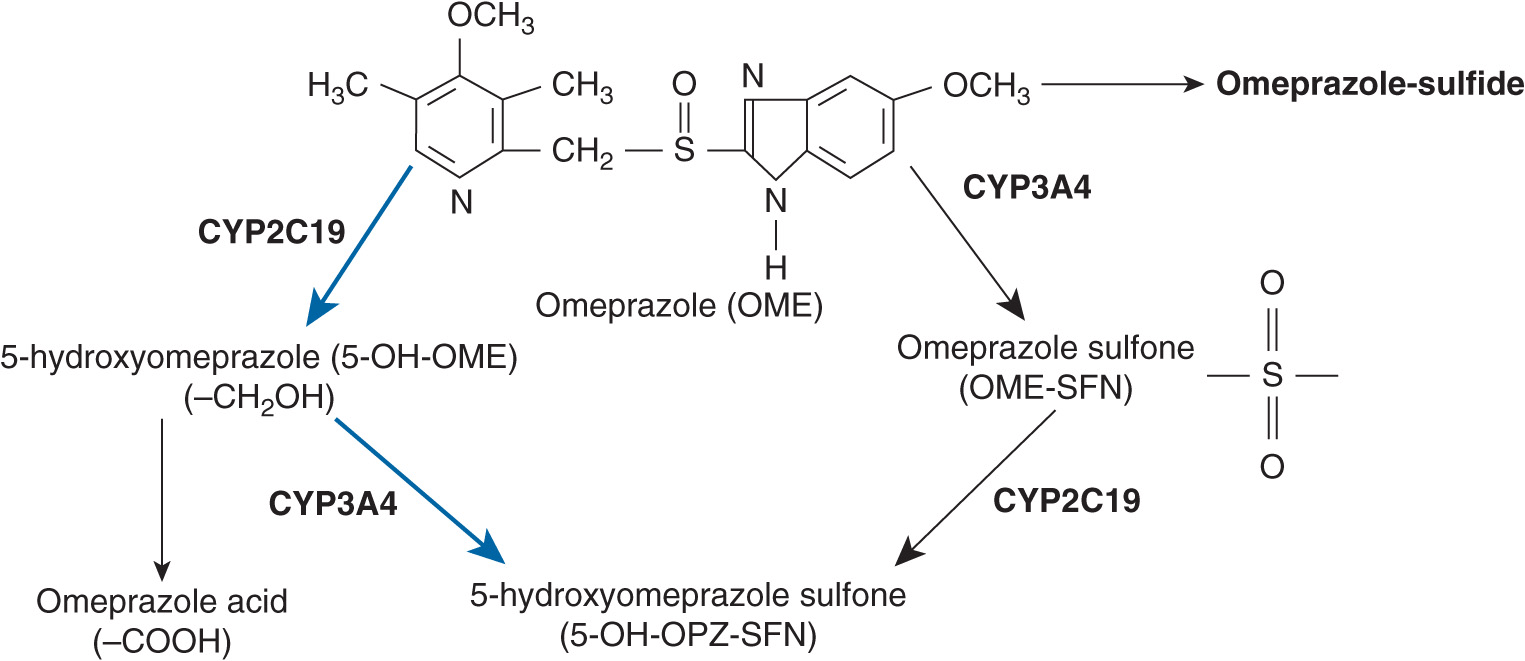
PPIs undergo the hepatic metabolism by the cytochrome P450 (CYP) system. The principal enzyme involved in the metabolism of PPIs is CYP2C19. CYP3A4 is also involved in PPI metabolism.10–14 For example, omeprazole, a representative and first clinically available PPI, is mainly metabolized by CYP2C19 to 5-hydroxyomeprazole, which is metabolized by CYP3A4 to 5-hydroxyomeprazole sulfone. Omeprazole is partially first metabolized by CYP3A4 to omeprazole sulfone, which is metabolized by CYP2C19 to 5-hydroxyomeprazole sulfone (Figure 17A–1). There are interindividual differences in the activity of CYP2C19. Recent reports have revealed that pharmacokinetics and pharmacodynamics of PPIs are affected by the CYP2C19 polymorphism.
FIGURE 17A–1 Metabolism of omeprazole (OME) in relation to cytochrome P450 (CYP) isoenzymes. Weight of arrows indicates the relative contribution of different enzyme pathways. OME is mainly metabolized by CYP2C19 to 5-hydroxyomeprazole (5-OH-OME), which is then metabolized to 5-hydroxyomeprazole sulfone (5-OH-OME-SFN). OME is also metabolized by CYP3A4 to omeprazole sulfone (OME-SFN), which is then metabolized by CYP2C19 to 5-OH-OME-SFN.
EFFECTS OF CYP2C19 POLYMORPHISM ON THE PHARMACOKINETICS AND PHARMACODYNAMICS OF PPIs
Polymorphism of Cytochrome P450 Enzymes
There are more than 20 kinds of CYP enzymes in the liver. These enzymes hydrolyze a variety of drugs. Among CYP enzymes, CYP1A2, 2C8, 2C9, 2C19, 2D6, and 3A4 are important in the metabolism of drugs in humans.15 Most of CYP450 enzymes show a genetic polymorphism associated with their enzyme activities (http://www.imm.ki.se/CYPalleles/default.htm). Plasma concentrations and effects of drugs metabolized by these enzymes differ among different individuals with their different enzyme activities genetically determined.
Genetic Differences in the Main PPI-Metabolizing Enzyme, CYP2C19
PPIs are mainly metabolized by CYP2C19 (Figure 17A–1). There are interindividual differences in the activity of this enzyme, which was first characterized by the racemic antiepileptic agent, mephenytoin, of which the S-enantiomer undergoes hydroxylation through CYP2C19.10,15,16 The polymorphism of this enzyme is classified into the three genotype groups: rapid metabolizer (RM) (=homozygous extensive metabolizer [homEM]), intermediate metabolizer (IM) (=heterozygous extensive metabolizer [hetEM]), and poor metabolizer (PM). In RMs, both of the alleles have no inactivating mutations and the enzyme can be generated from both of the nonmutated alleles. In IMs, the one allele has the mutation in the coding region of CYP2C19 (see Chapter 6). However, the other allele has no mutation and normal enzyme can be generated from this allele. In PMs, both of the alleles have mutations in the CYP2C19 genes, and, therefore, normal enzyme cannot be generated from any of the two mutated alleles, resulting in the deficiency of the enzyme activity.
There are interethnic differences in the frequencies of PMs of this enzyme: 2.5% in the white Americans, 2.0% in the African Americans, 3.5% in the white Europeans, 4.8% in Shona Zimbabweans, 19.8% in the Chinese-Han population, 13.4% in the Chinese-Bai population, 12.6% in the Korean population, and 18.0–22.5% in the Japanese population.17–24
Various genetic mutations involved in the CYP2C19 polymorphism have been discovered from ethnically different populations (http://www.imm.ki.se/CYPalleles/cyp2c19.htm). However, the PM-related CYP2C19 polymorphism of Japanese and Caucasian people essentially can be explained by two point mutations generating inactive alleles, that is, CYP2C19*2 (exon 5 mutation) and CYP2C19*3 (exon 4).22,25,26
Effect of CYP2C19 Genotypic Differences on the Pharmacokinetics and Pharmacodynamics of PPIs
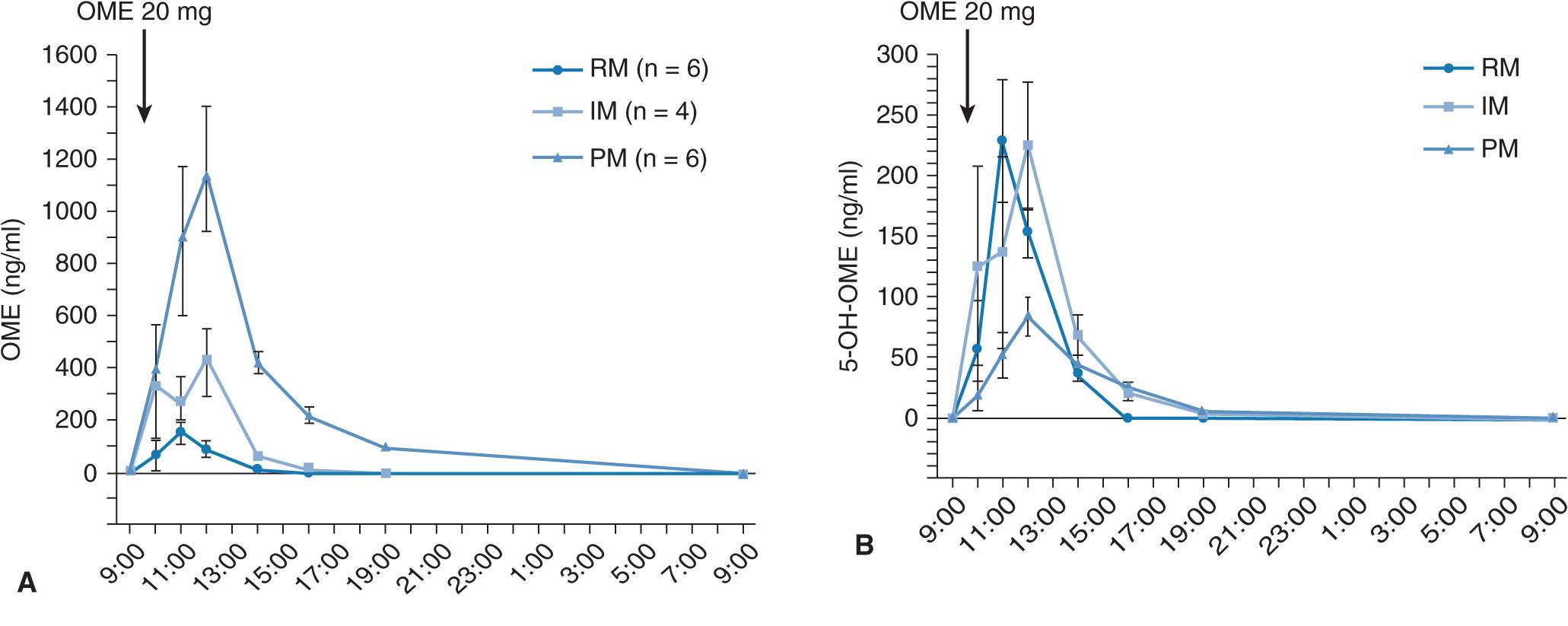
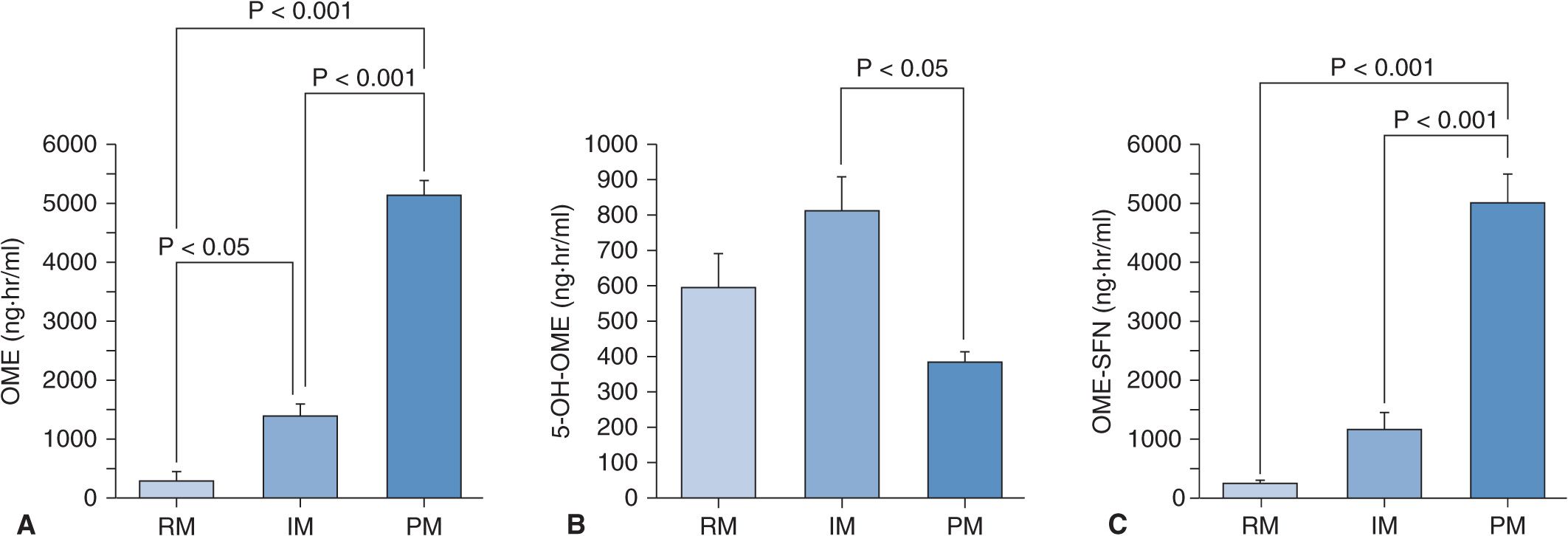
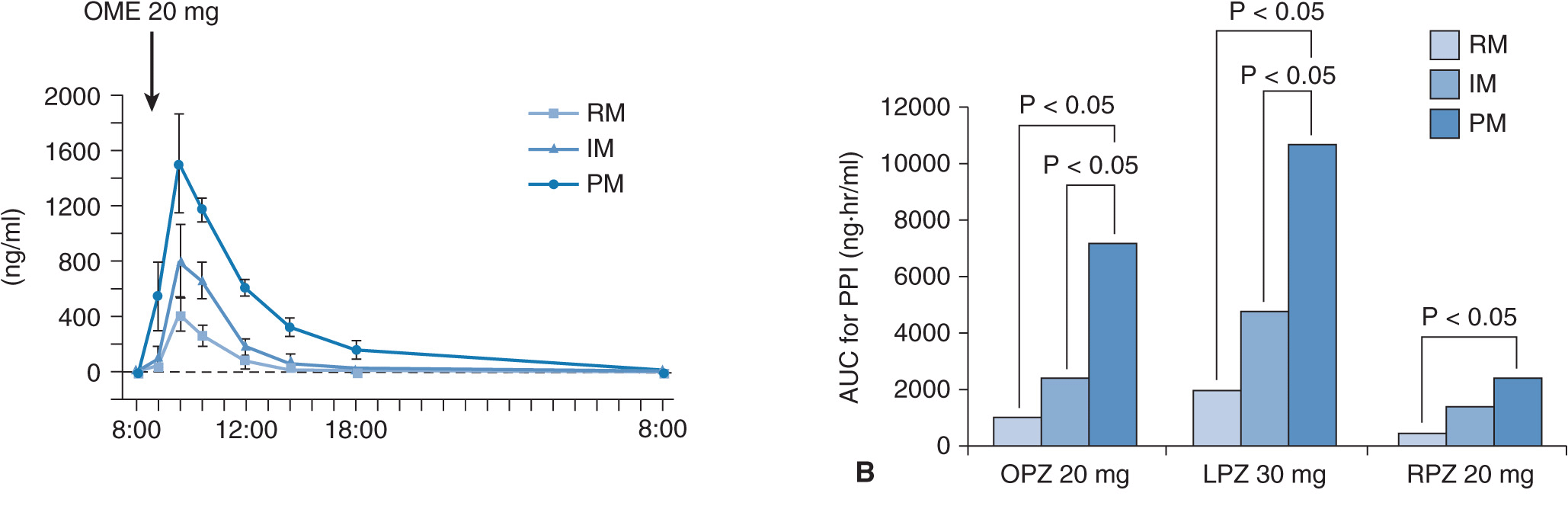
When 20 mg of omeprazole (the active drug) is given as a single dose, plasma omeprazole concentrations differ among the three different CYP2C19 genotype (RM, IM, and PM) groups (Figure 17A–2A).27 Plasma omeprazole concentrations in the PM group are sustained for a long time after dosing. On the other hand, plasma concentrations of 5-hydroxyomeprazole, which is formed from omeprazole via CYP2C19, in the PM group are lower than those in the RM and IM groups. Hydroxylation of omeprazole in PMs is mediated by CYP3A4.28 In PMs, the sulfoxidation of omeprazole is the main metabolic pathway and omeprazole sulfone cannot be metabolized to 5-hydroxyomeprazole sulfone by CYP2C19 in the PM group, because PMs lack CYP2C19. Therefore, plasma omeprazole sulfone concentrations are sustained for a long time after dosing in the PM group (Figure 17A–2C). The mean value for the area under the plasma concentration–time curves (AUC) of omeprazole in the PM group is about 13 times as high as that of the RM group (Figure 17A–3A). There are also significant differences in the two omeprazole metabolite, 5-hydroxyomeprazole and omeprazole sulfone, concentrations among the three different CYP2C19 genotype groups (Figures 17A–3B and C).
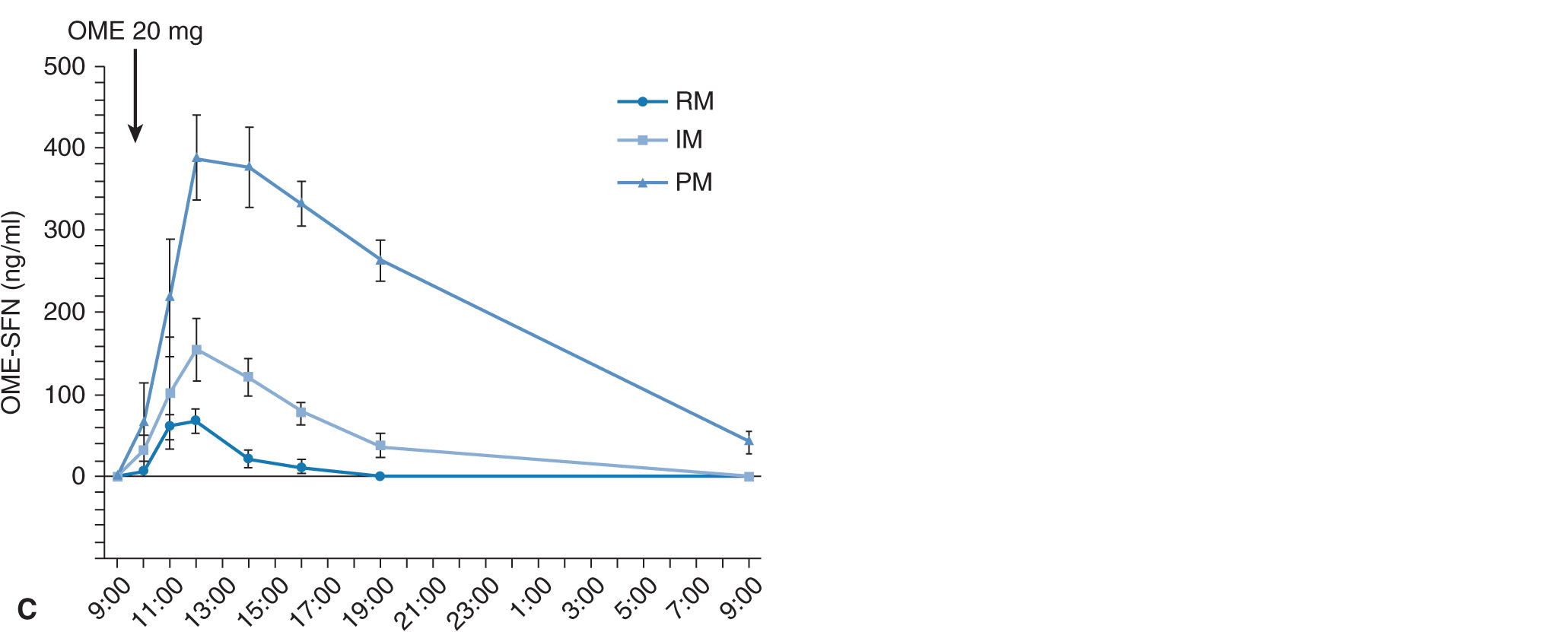
FIGURE 17A–2 Mean (±SE) plasma concentrations of omeprazole (OME) (A), 5-hydroxyomeprazole (5-OH-OME) (B), and omeprazole sulfone (OME-SFN) (C) as a function of CYP2C19 genotype. (A) Plasma concentration of OME was highest in the PM group, intermediate in the IM group, and lowest in the RM group. (B) The peak concentration (Cmax) of 5-OH-OME was observed first in the RM group. Plasma concentration of OH-OME was lowest in the PM group. (C) Plasma concentration of OME-SFN was highest in the PM group, intermediate in the IM group, and lowest in the RM group. Abbreviations: RM, rapid metabolizer; IM, intermediate metabolizer; PM, poor metabolizer.
FIGURE 17A–3 Means (±SE) of the areas under the plasma concentration–time curves (AUC) for omeprazole (OME) (A), 5-hydroxyomeprazole (5-OH-OME) (B), and omeprazole sulfone (OME-SFN) (C) as a function of CYP2C19 genotype when 20 mg of omeprazole is once dosed. (A) The mean AUC for OME was highest in the PM group, intermediate in the IM group, and lowest in the RM group. (B) The mean AUC for 5-OH-OME in the PM group was lowest of the three groups. (C) The mean AUC for OME-SFN was highest in the PM group, intermediate in the IM group, and lowest in the RM group. See abbreviations in the legend of Figure 17A–2.
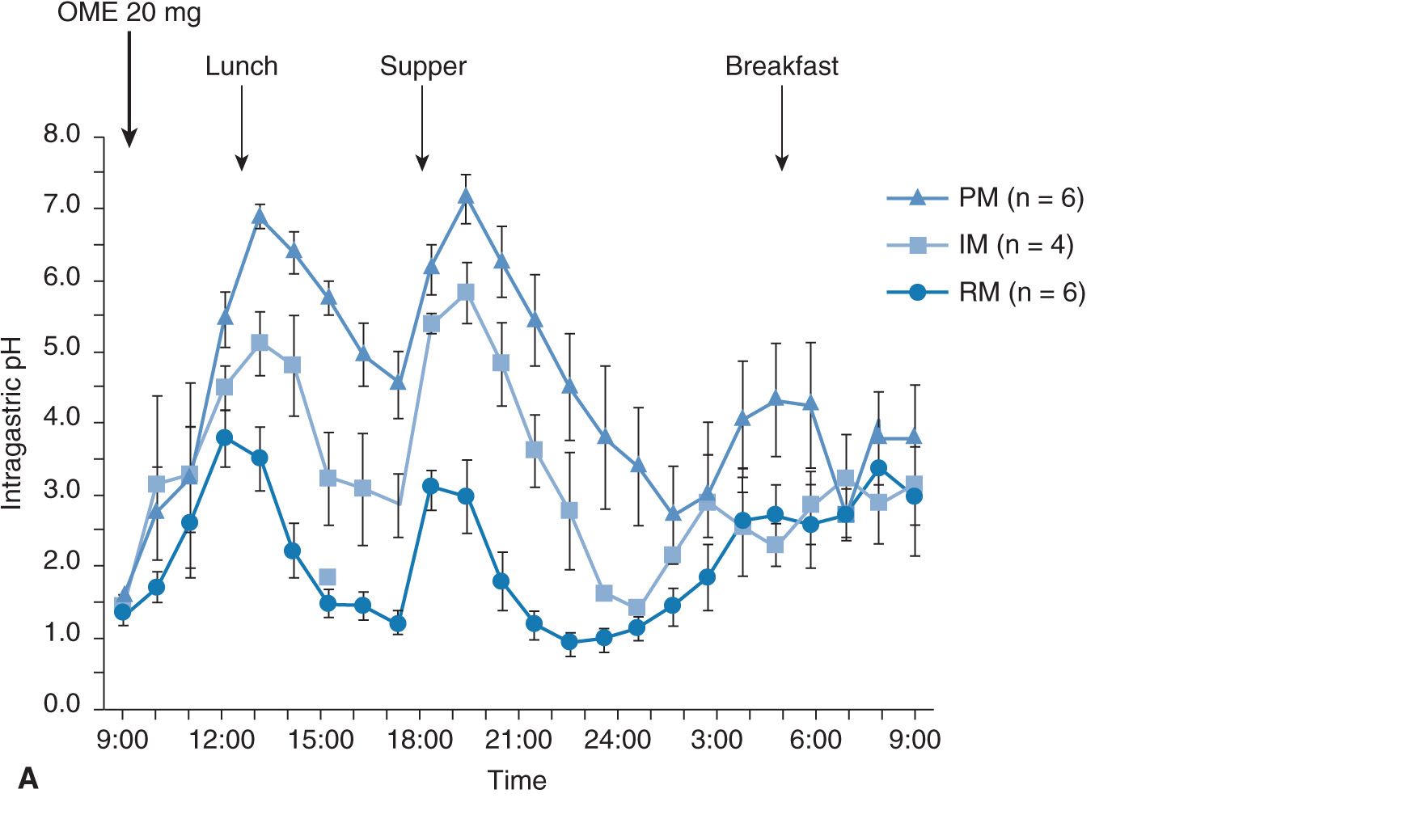
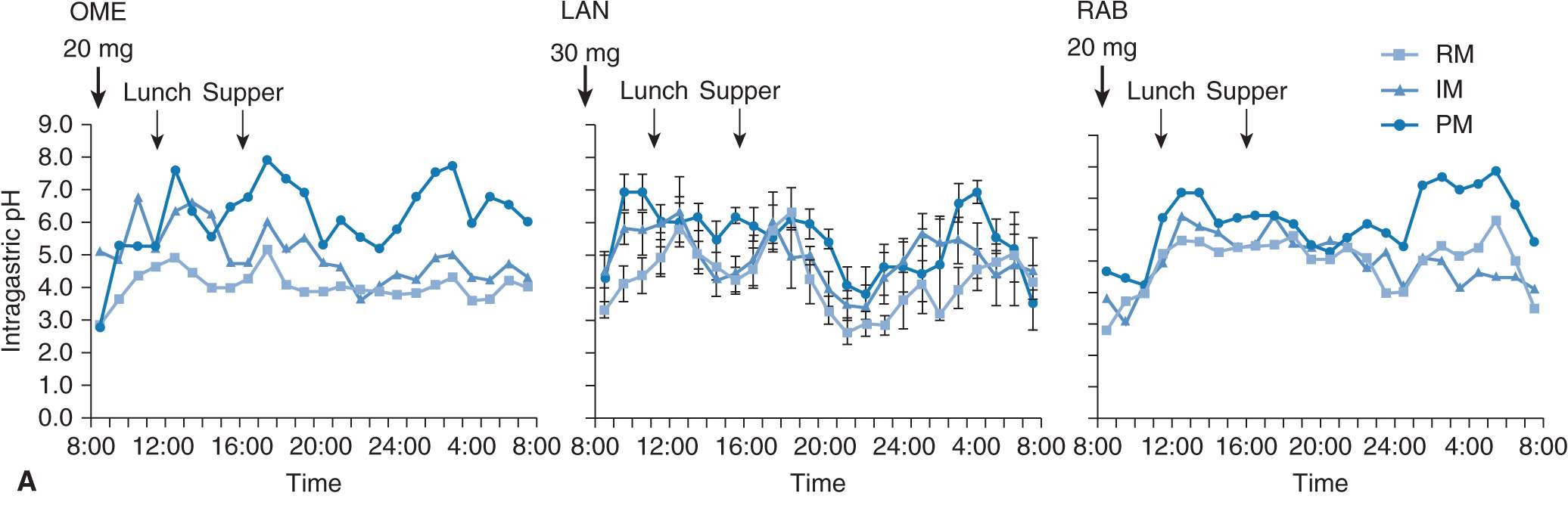
The intragastric pH profile also differs among the three different genotype groups (Figure 17A–4A). The mean 24-hour intragastric pH level in the RM group is the lowest, that of the IM group comes next, and that in the PM group is highest (Figure 17A–4B). The acid inhibition achieved by omeprazole in the RM group seems insufficient under the so-called standardized dosing scheme,27,29 as discussed below.
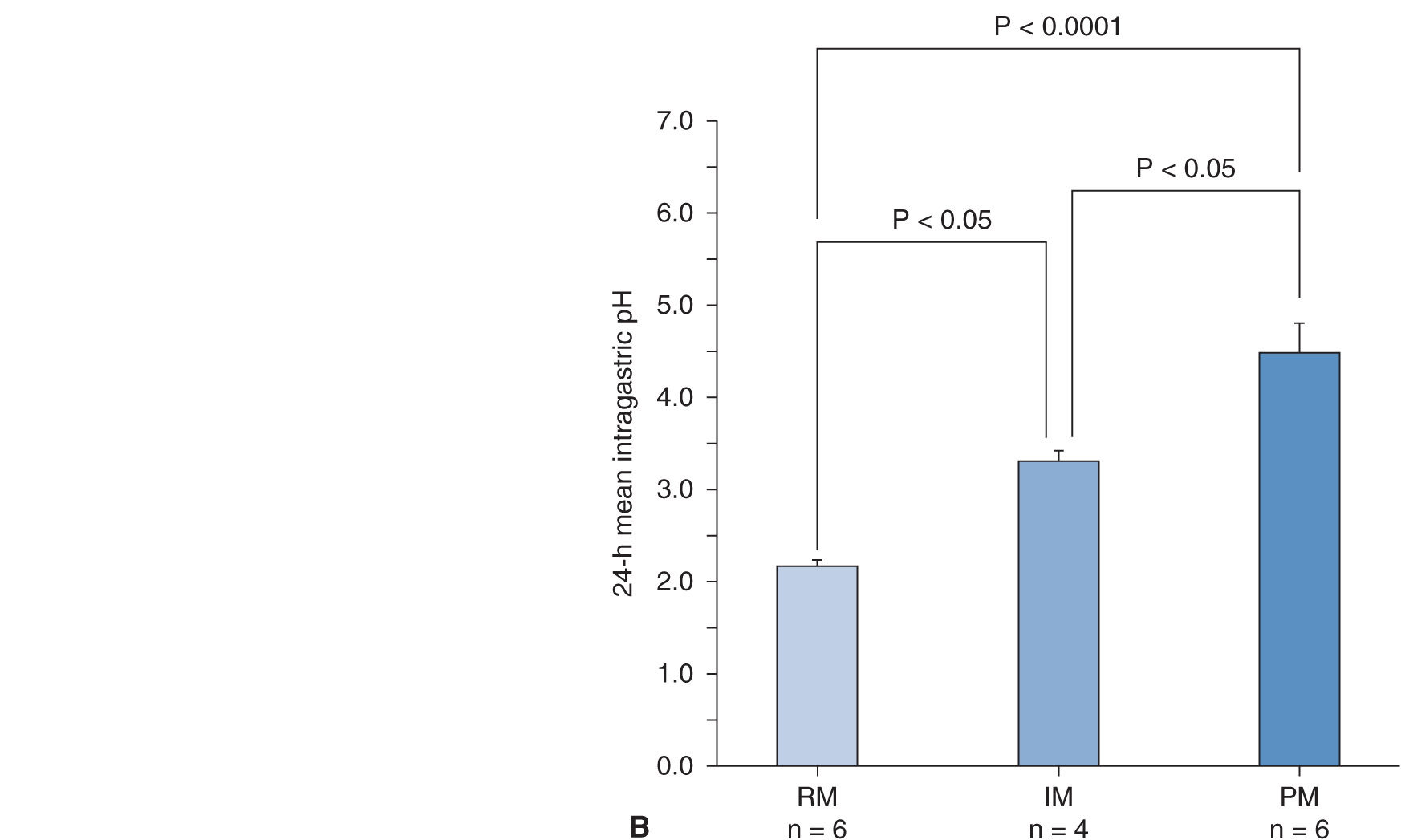
FIGURE 17A–4 Profiles of intragastric pH values (A) and the 24-hour mean intragastric pHs (B) as a function of CYP2C19 genotype status for 20 mg of omeprazole (OME) dosing. The mean intragastric pH values differed significantly ![]() among the three groups when 20 mg of OME was once dosed.
among the three groups when 20 mg of OME was once dosed.
Plasma concentration–time curves after oral dosing of 20 mg of omeprazole, 30 mg of lansoprazole, and 20 mg of rabeprazole for 8 days as a function of CYP2C19 genotype status are also affected by CYP2C19 genotype status (Figure 17A–5A). The mean values for the AUCs of omeprazole, lansoprazole, and rabeprazole in subjects with RM, IM, or PM genotype of CYP2C19 are summarized in Figure 17A–5B. Intragastric pH profiles after repeated dosing of 20 mg of omeprazole, 30 mg of lansoprazole, or 20 mg of rabeprazole for 8 days are also affected by CYP2C19 genotype status (Figure 17A–6A),30–32 although the difference in acid inhibitory effect of a PPI among different CYP2C19 genotype statuses becomes smaller by the repeated dosing of a PPI (compare the pH data in Figure 17A–6A with those in Figure 17A–4A). The differences in acid inhibition by a PPI among the different CYP2C19 genotype groups are considered to come from different plasma concentrations among the different genotype groups: profound acid inhibition in PMs is considered to be derived from higher plasma PPI concentrations for a longer period in PMs and low acid inhibition in RMs is supposed to be ascribable to low plasma PPI concentrations in them.
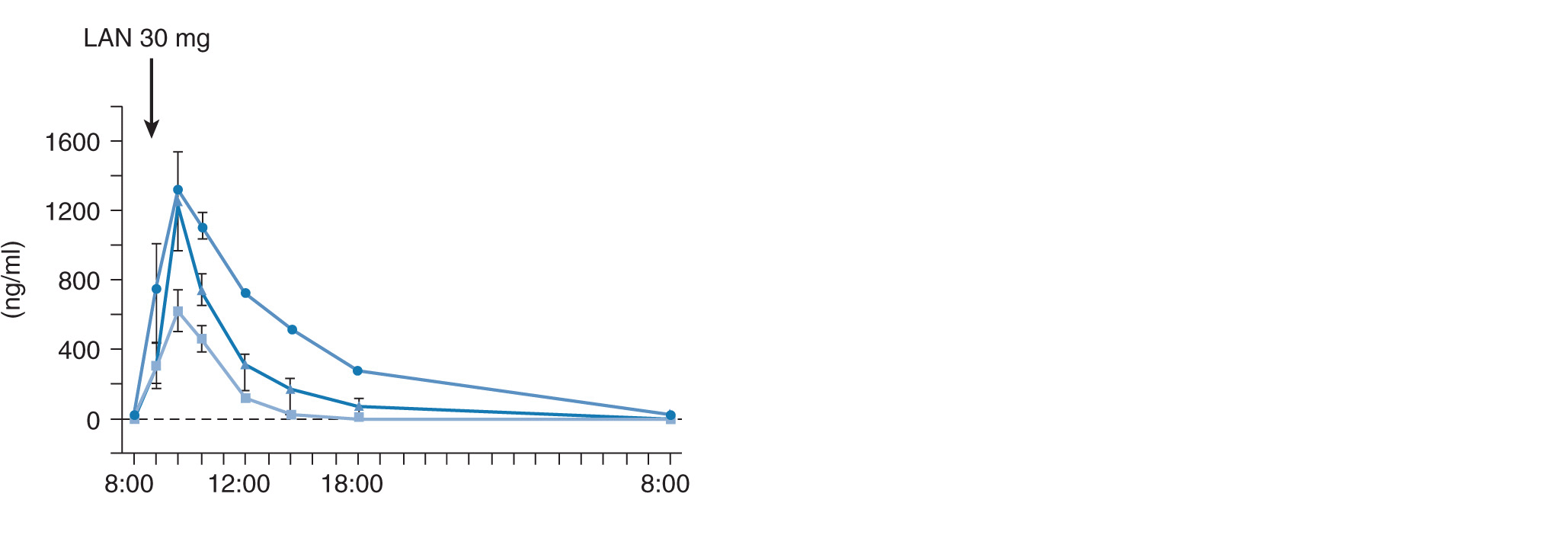
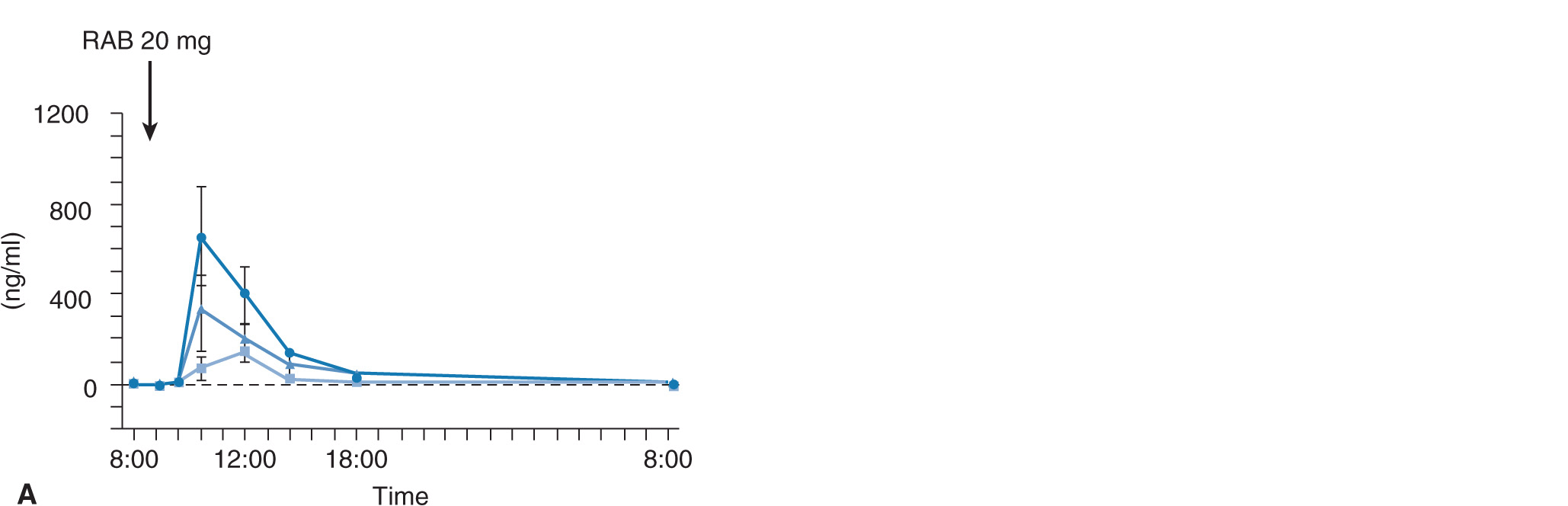
FIGURE 17A–5 (A) Mean (±SE) plasma levels of 20 mg of omeprazole (OME), 30 mg of lansoprazole (LAN), and 20 mg of rabeprazole (RAB) as a function of CYP2C19 genotype for 8 days. Plasma concentrations of OME, LAN, and RAB were highest in the PM group, intermediate in the IM group, and lowest in the RM group. (B) Area under the plasma concentration–time curves for omeprazole (OME), lansoprazole (LAN), and rabeprazole (RAB) as a function of CYP2C19 phenotype. AUC values of OME, LAN, and RAB were highest in the PM group, intermediate in the IM group, and lowest in the RM group.
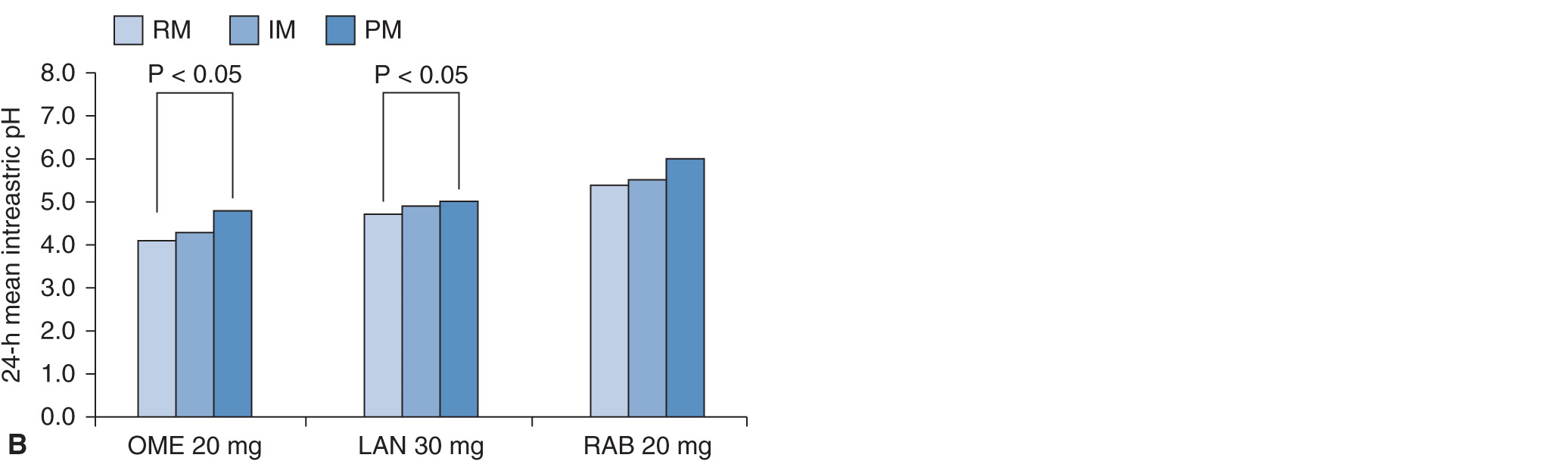
FIGURE 17A–6 (A) Mean (±SE) intragastric pH values as a function of CYP2C19 genotype status for 20 mg of omeprazole (OME), 30 mg of lansoprazole (LAN), or 20 mg of rabeprazole (RAB) dosing for 8 days. (B) Median of the 24 hour-mean intragastric pH values as a function of CYP2C19 phenotype status for 20 mg of omeprazole (OME), 30 mg of lansoprazole (LAN), or 20 mg of rabeprazole (RAB) dosing. The 24-hour mean intragastric pH value was highest in the PM group, intermediate in the IM group, and lowest in the RM group. Statistical significant difference was observed between the RM group and the PM group when 20 mg of OME or 30 mg of LAN was dosed. See the legend of Figure 17A–2 for the abbreviations.
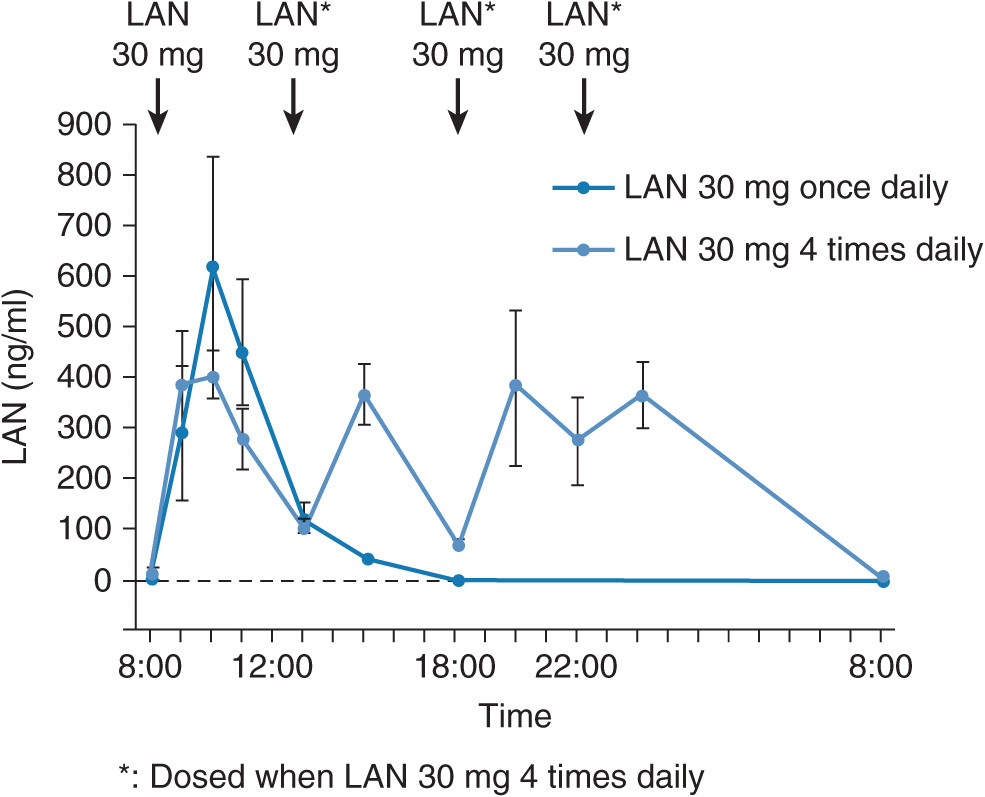
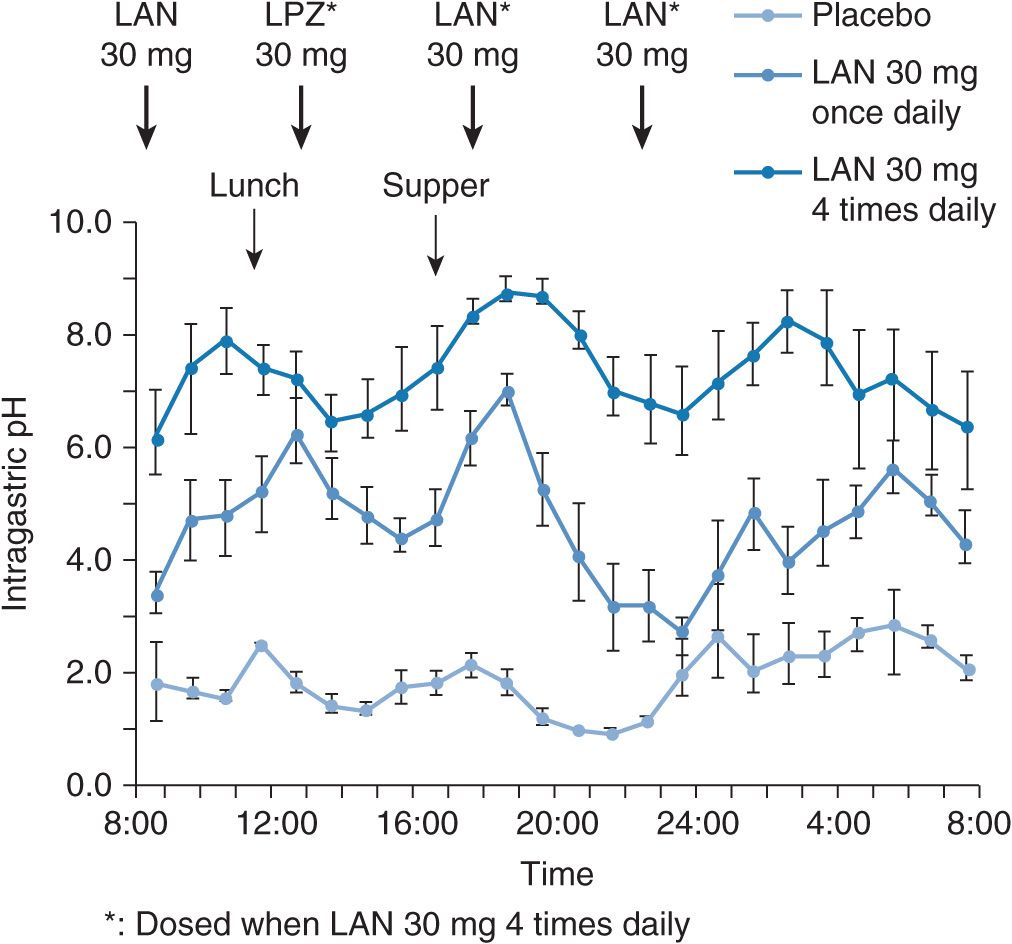
However, when 30 mg of lansoprazole is dosed four times daily in order to sustain the plasma lansoprazole concentrations all day long (Figure 17A–7), a complete acid inhibition can be achieved even in RMs (Figure 17A–8). Because of the irreversible blockade of the proton pump and the involvement of metabolites in the effects of PPIs, the time course and extent of acid suppression is not directly linked to plasma concentrations. Interestingly, the peak plasma concentration (Cmax) of lansoprazole in RMs when 30 mg of lansoprazole is dosed four times daily is not increased in comparison with the case of once-daily dosing of 30 mg of lansoprazole (Figure 17A–7) and is not as high as that observed in PMs, although sufficient acid inhibition is achieved in RMs when lansoprazole was dosed four times daily. This indicates that the faster elimination of lansoprazole rather than its lower Cmax is the primary reason for the insufficient acid inhibition in RMs. In other words, the acid inhibitory effect of PPIs depends more on the plasma elimination half-life time (t1/2) than on Cmax. Therefore, the dosing scheme of a PPI is a key point to attain an appropriate intragastric pH level and should be determined on the basis of the individual CYP2C19 genotype status for a sufficient acid inhibition.
FIGURE 17A–7 Mean (±SE) plasma concentration–time curves for lansoprazole (LAN) after the final dosings of 30 mg of LAN once daily and 30 mg of lansoprazole four times daily for 8 days in the five RMs. By four times daily dosing of 30 mg of LAN, plasma levels of LAN are sustained during each of the dosing intervals in the five RMs.
FIGURE 17A–8 Mean (±SE) intragastric pH values versus time course after the last dosings when placebo, 30 mg of LAN once daily, and 30 mg of LAN four times daily were dosed. Although the pH values were decreased to the levels less than 4.0 for several hours during nocturnal time when LAN 30 mg was dosed once daily for 8 days, no such decrease in the pH levels was observed when LAN 30 mg was dosed four times daily for 8 days. Complete acid inhibition (i.e., intragastric pH around 7.0) can be achieved by LAN 30 mg four times daily dosing for 8 days.
EFFECT OF CYP2C19 POLYMORPHISM ON GERD TREATMENT BY A PPI
GERD is a common disorder, which represents a major indication for PPIs. The cure rates of GERD attained by PPIs are around 90%, while there are some patients who do not respond to the usual standard dose of a PPI (e.g., 20 mg of omeprazole or 30 mg of lansoprazole).33–35 Recently, one of the reasons for GERD refractory to the PPI treatment has been shown to be associated with the metabolism of a PPI as discussed below.
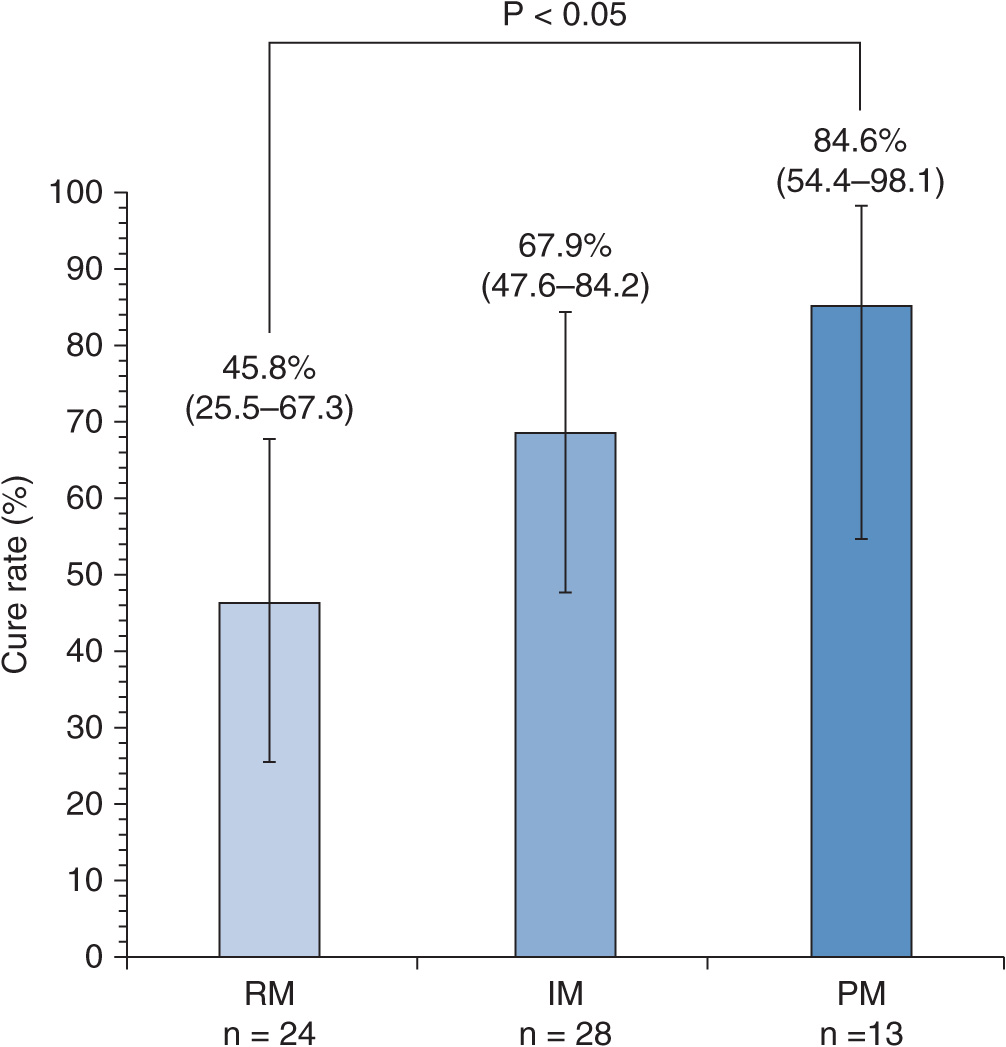
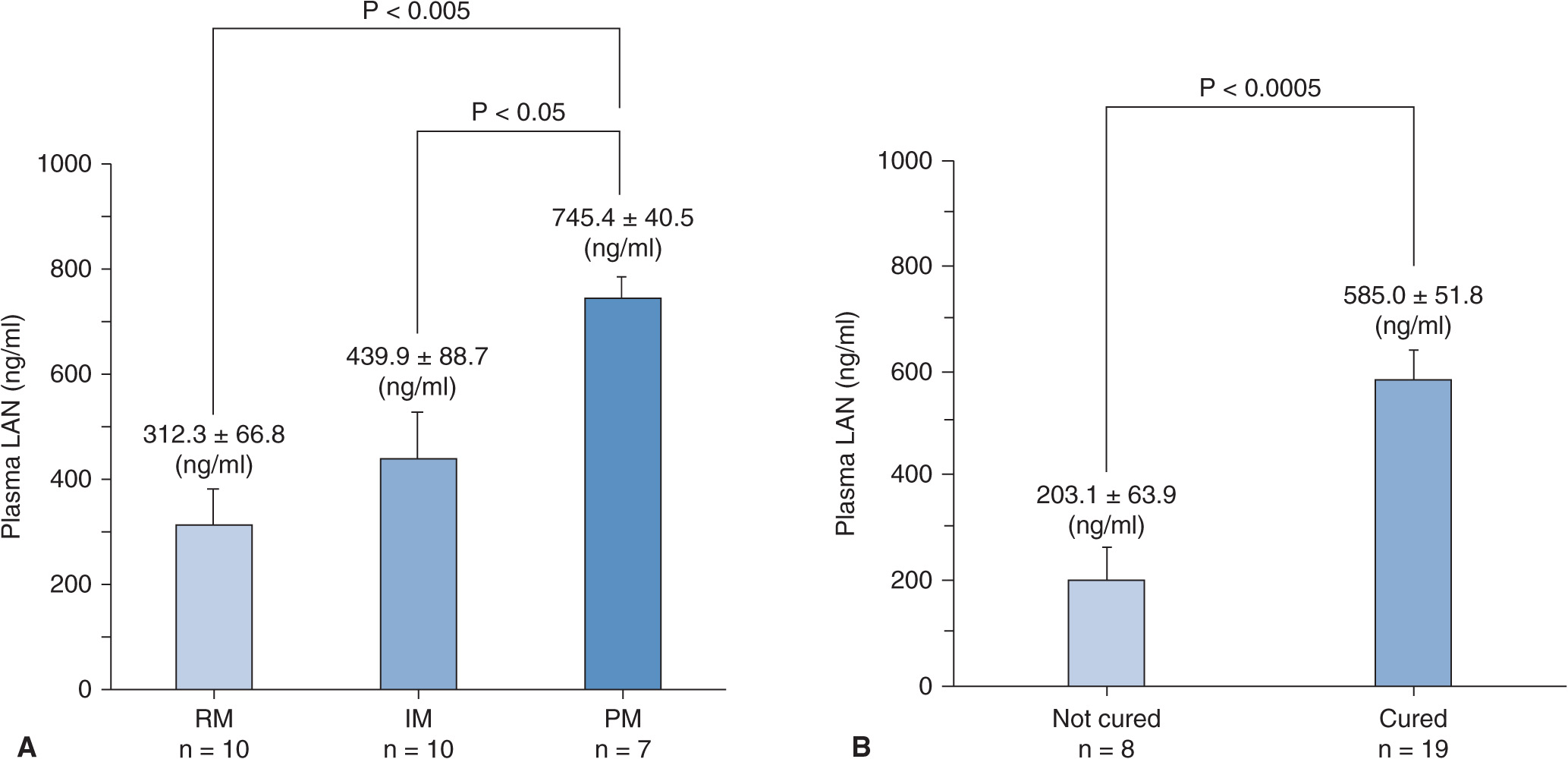
When 30 mg of lansoprazole was dosed in GERD patients positive for mucosal breaks (grades A–D in Los Angeles classification) for 8 weeks, cure rates of mucosal breaks significantly depended on CYP2C19 genotype status.36 The cure rate of mucosal breaks in the RM group was lowest, that in the IM group came next, and that in the PM group was highest (Figure 17A–9), with the observation that the cure rate of grade C or D of GERD in patients with the RM genotype of CYP2C19 was dramatically low (![]() , CI: 0.4–64.1%).36 Plasma lansoprazole concentrations at 3 hours after the last dose depended on the CYP2C19 genotype status (Figure 17A–10A) and were associated with cure rates of mucosal breaks of GERD. The mean plasma lansoprazole concentration in the successfully treated group was significantly higher than that in the unsuccessfully treated group (Figure 17A–10B). Similarly, Kawamura et al.37 also reported that the cure rate of mucosal breaks treated with lansoprazole 30 mg daily for 8 weeks in the RM patients was lowest of the three different CYP2C19 genotype groups. Recurrence of GERD symptoms during the maintenance therapy with a PPI after cure of mucosal breaks is also affected by CYP2C19 genotype status.38 Acid inhibition achieved by a daily dose of 30 mg of lansoprazole in the PM genotype group appears to be clinically sufficient for GERD treatment, and those in IMs and RMs might be insufficient in several cases.
, CI: 0.4–64.1%).36 Plasma lansoprazole concentrations at 3 hours after the last dose depended on the CYP2C19 genotype status (Figure 17A–10A) and were associated with cure rates of mucosal breaks of GERD. The mean plasma lansoprazole concentration in the successfully treated group was significantly higher than that in the unsuccessfully treated group (Figure 17A–10B). Similarly, Kawamura et al.37 also reported that the cure rate of mucosal breaks treated with lansoprazole 30 mg daily for 8 weeks in the RM patients was lowest of the three different CYP2C19 genotype groups. Recurrence of GERD symptoms during the maintenance therapy with a PPI after cure of mucosal breaks is also affected by CYP2C19 genotype status.38 Acid inhibition achieved by a daily dose of 30 mg of lansoprazole in the PM genotype group appears to be clinically sufficient for GERD treatment, and those in IMs and RMs might be insufficient in several cases.
FIGURE 17A–9 Cure rates of GERD with a daily dose of 30 mg of lansoprazole for 8 weeks in the different CYP2C19 genotype groups. Bars indicate 95% confidence intervals (95% CI). There was a significant difference in the cure rates among the three different CYP2C19 genotype groups. The cure rate in the RM group was lowest, that in the IM group came next, and that in the PM group was the highest. P-Value was derived from Fisher’s exact test.
FIGURE 17A–10 The mean ± SE concentrations of plasma lansoprazole (LAN) at 3 hours after the final 30-mg dose of LAN in the different CYP2C19 genotype groups (A) and in the unsuccessfully or successfully treated groups (B). P-Values were derived from Scheffe’s multiple comparison test (A) and Student’s t-test (B). Significant differences in the mean plasma LAN concentrations among different CYP2C19 genotype groups were observed. The mean plasma LAN concentration in the RM group was lowest, that in the IM group came next, and that in the PM group was highest of the three genotype groups (A). The mean plasma LAN concentration in the group without cure of GERD was significantly lower than that in the successfully treated group (B).
Nocturnal acid breakthrough (NAB), which is defined as an intragastric pH lower than 4 lasting for more than 1 hour during the overnight period, is now considered as one of the factors associated with the success or failure of treatment of GERD with PPIs.39–42 Interestingly, the frequency of NAB depends on the CYP2C19 genotype status and NAB is most frequently seen in subjects with the RM genotype pattern of CYP2C19,31,32 resulting in the lowest cure rate of GERD of the RM patients among the three different genotype groups.36,37
On the basis of the above discussion, an increased dose of a PPI is recommended for the RM GERD patients who are refractory to the treatment with a usual standard dose of a PPI. In fact, a usual dose of a PPI, such as 20 mg of omeprazole daily, was reported to be therapeutically insufficient for some patients in western countries, whereas an increased dose of omeprazole, up to 80 mg daily, successfully treated such patients,43 although the majority of westerners have the RM genotype of CYP2C19.44 A more frequent daily dosing of lansoprazole (e.g., 30 mg four times a day) can achieve sufficient acid suppression (i.e., intragastric pH around 7) in the RM genotype group as noted above.32 Therefore, an increased dose of PPIs might be one of the therapeutic strategies for the treatment of GERD refractory to the usual dose of a PPI in patients with the RM genotype of CYP2C19.
It has been reported that the acid inhibitory effect of histamine 2 (H2) receptor antagonists, such as famotidine, is not affected by the CYP2C19 genotype status and superior to that of lansoprazole in the RM patients during the nighttime.31 Adding an evening dose of an H2 receptor antagonist to a morning dose of a PPI has also been reported to be effective for the control of NAB in individuals who are resistant to a usual PPI treatment.45–47 Therefore, concomitant treatment with a PPI plus an H2 receptor antagonist appears to be another therapeutic strategy for RM patients with GERD refractory to treatment with a usual constant dose of a PPI alone.
Accordingly, CYP2C19 genotype status is considered as one of the predictable determinants for the results of a PPI-based GERD therapy. If CYP2C19 genotype status is determined before treatment, an optimal dose of a PPI can be prescribed, based on this predetermined pharmacogenomic status. Therefore, the individualized therapeutic strategy based on the individual CYP2C19 genotype status should be conducted in such a therapeutic manner as performed with an increased dose of a PPI or adding an H2 receptor antagonist, particularly in the patients with the RM genotype of CYP2C19 and severe GERD (i.e., grade C or D). This predetermined therapeutic strategy should be expected to increase the cure rates achieved by the initial treatment for GERD.
THE EFFECTS OF CYP2C19 POLYMORPHISM ON PPI-BASED ERADICATION THERAPY OF H. PYLORI
H. pylori is clearly associated with the pathogenesis of a variety of upper gastrointestinal disorders, such as peptic ulcer, mucosa-associated lymphoid tissue lymphoma, and gastric cancer.48–51 Eradication of this pathogen is now an important treatment strategy for the cure of these diseases. Current regimens for the eradication of H. pylori consist of a PPI plus one or two antibacterial agents, such as amoxicillin, clarithromycin, and metronidazole.
The roles of PPIs in an H. pylori eradication therapy are as follows. First, PPIs make antibiotics more stable and bio-available in the stomach by raising intragastric pH to neutral levels.52 Second, neutralization of intragastric pH levels allows H. pylori to shift into the growth phase and thus they become more sensitive to antibiotics, such as amoxicillin.53–55 Third, suppression of acid secretion by a PPI increases the concentration of an antibiotic, such as amoxicillin, in the stomach.56 Fourth, PPIs per se have an anti–H. pylori effect.57 It is assumed that the weaker the effect of a PPI is, the lower the eradication rate is, and that the more potent the effect of a PPI is, the higher the eradication rate is. As a matter of fact, the cure rates achieved by treatment with two antibacterial agents (amoxicillin plus clarithromycin or clarithromycin plus metronidazole) without a PPI were significantly lower than those achieved by the treatment with the same two antibacterial agents plus omeprazole, a representative PPI.58 Therefore, coadministration of a PPI with an antibiotic or antibiotics is essential for the treatment of H. pylori infection.
DUAL PPI/AMOXICILLIN ERADICATION THERAPY FOR H. PYLORI INFECTION IN RELATION TO CYP2C19 POLYMORPHISM
Dual Omeprazole/Amoxicillin Therapy
The eradication rates for H. pylori by dual omeprazole/amoxicillin (omeprazole 20 mg once daily plus amoxicillin 500 mg four times daily for 2 weeks) are approximately 30% in the RMs, 60% in the IMs, and 100% in the PMs (Figure 17A–11).59 Differences in plasma omeprazole concentrations among the different CYP2C19 genotype groups are assumed to reflect the different eradication rates among the correspondent genotype groups. Aoyama et al.60 reported that eradication rates by dual omeprazole/amoxicillin therapy (omeprazole 40 mg plus amoxicillin 2,000 mg daily for 1 week) were 33% in the RMs, 30% in the IMs, and 100% in the PMs. These two reports59,60
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree