The qualifying statement leads to the following extended definition: ‘If all components are in the same physical state, the component present in the greatest proportion is considered the solvent for the system.’ For example, ethanol can dissolve in water or water can dissolve in ethanol. The identification of which component is the solvent depends on the relative weights (not usually volumes for alcoholic solutions) of each. If the amount of water is greater than that of ethanol, water is considered the solvent and ethanol the solute. If ethanol is present in a greater amount than that of water the converse will be true. The extent to which a solute can dissolve in a solvent is its solubility in that particular solvent. The extent and rate of solution are two different concepts, but can be related.
Solubility and dissolution rate will be discussed in Chapter 3. At this point, the variety of solvents available, and their different means of interacting with solutes, are important to understand. Generally, most solvents used in drug delivery systems are polar. Therefore, as described in Chapter 1, most of our solvents function using repulsive and attractive intermolecular interactions – van der Waals forces (which in this text include all three of the forces, dipole–dipole, dipole–induced dipole, and induced dipole–induced dipole) – as well as ion–dipole, ion–induced dipole, and electrostatic repulsive forces (see Chapter 1). Solvent actions occur as a result of repulsive and attractive forces at work, which produces a solution. Solutions will be further discussed in Chapter 3.
Why are solvents useful as part of drug delivery systems? Obviously, dissolution of drugs and other components of drug delivery systems is a primary physical pharmacy function. In addition, if a liquid delivery system is desired, the proper solvent must be used. Solvents also can influence palatability of oral systems and improve (or weaken) product stability. However, since many drugs need to be delivered in solution, the choice of solvent is often tantamount to creating successful delivery systems.
In Chapter 1 it was emphasized that presence or absence of a dipole (polarity) is an important property for substances because it dictates much of the intermolecular interaction that occurs among molecules. Therefore, it should not be surprising that solvents can be categorized based on this property. Solvents comprise three types: polar, semipolar, and nonpolar. When selecting the best solvent for a drug delivery system, a solvent from the appropriate category that best matches the property of the solute (the material being solvated) is chosen, as much as is practical.
Some solutes ionize when in aqueous solution, while others do not. Some are lipophilic, some hydrophilic. Polar solvents are strong dipolar molecules that often employ hydrogen bonding as an interaction type. Polar solvents also sometimes act by breaking covalent bonds of a solute, causing solute ionization. Polar solvents, including among others, water and alcohols, are the most prominent solvents used in drug delivery systems. The most common alcohols used as solvents include ethanol, isopropyl alcohol, glycerin, and propylene glycol. Again, some of these are useful for external applications but may be useful for internal applications only in small amounts, if at all, due to toxicity problems. Common solutes for which polar solvents are used include other polar solvents (such as alcohols, aldehydes, and ketones), sugars, and other compounds with –OH groups. Polar solvents typically have dielectric constants greater than 50 (Figure 2.1).
Key Point
Polar solvents are those whose dielectric constants are 50 and greater.
Semipolar solvents typically are strong dipolar molecules that do not form hydrogen bonds but can induce polarity in nonpolar molecules (D–I and I–I; see Chapter 1) – both solutes and solvents. Solvents are considered ‘semipolar’ when their dielectric constants are between 20 and 50. When used for induction of a nonpolar solvent, such as benzene, the semipolar solvent is acting as an intermediate solvent. An example of this is when acetone (ε ~ 21) increases the solubility of ether (ε ~ 4) in water (ε ~ 80). Semipolar solvents include acetone, aldehydes and other ketones, some esters, and nitro-compounds (Figure 2.2).
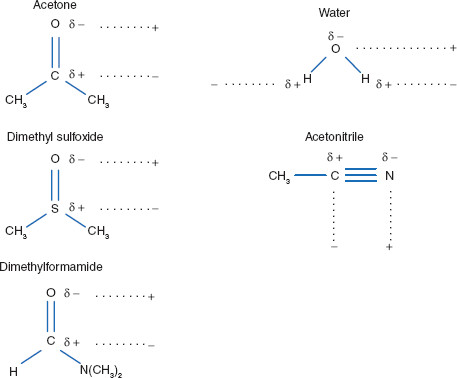
Figure 2.1 Actions of polar solvents
Polar solvents are those whose dielectric constants are 50 and greater. Polar solvents utilize permanent dipole moments and hydrogen bonding to enable interaction with electron-rich regions of solutes.
Key Point
Semipolar solvents are those whose dielectric constants are between 20 and 50.
Nonpolar solvents possess little or no dipolar character. Although they tend to be unable to independently form dipoles (i.e., depend on induced dipoles) they can utilize induced dipole–induced dipole (ID–ID) interactions for dissolving appropriate solutes. Nonpolar solvents have dielectric constants between 1 and 20, and include fixed oils, carbon tetrachloride, and chloroform among others (Figure 2.3). Ionic and polar solutes have little-to-no solubility in nonpolar solvents. However, oils, fats, and fatty acids dissolve well in nonpolar solvents.
Key Point
Nonpolar solvents are those whose dielectric constants are between 1 and 20.
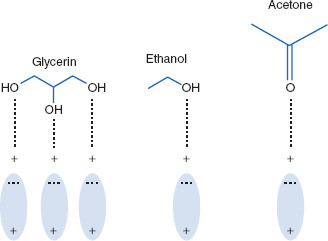
Figure 2.2 Semipolar solvents and induced polarity
Common semipolar solvents, with dielectric constants, include ethanol (25), glycerin (46), and acetone (21). Semipolar solvents are not as polar as solvents like water but can still induce dipoles in other molecules, solvents, or solutes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


