INTRODUCTION: THE CENTRAL NERVOUS SYSTEM
The central nervous system (CNS) comprises the brain and spinal cord and is the most complex organ system in the human body. The CNS differs from other organ systems in the variety of functions that it provides and in the localization of these functions to specialized areas of the CNS. The localization of specialized functions means that a relatively small, focal lesion in the CNS can produce a profound deficit, for example, loss of speech. This localization also results in the various populations of neurons within the CNS having unique capabilities and also unique vulnerabilities to disease. For example, Parkinson disease (PD) preferentially affects the neurons of the substantia nigra in the brain stem, while Alzheimer disease (AD) preferentially affects the neurons of the cerebral cortex.
CNS HISTOLOGY AND COMMON CELLULAR RESPONSES TO INJURY
QUICK REVIEW
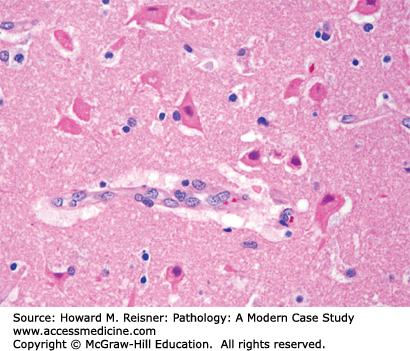
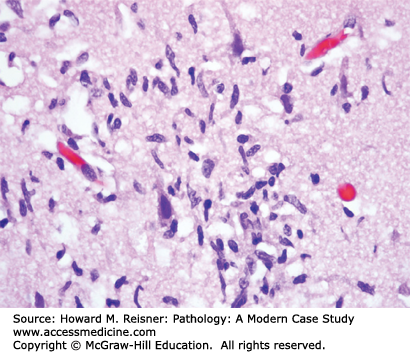
Neurons (Figure 21-1) are the principal cell type within the nervous system. Acute neuronal injury is most often due to ischemia, but there are many other causes, including trauma, infections, toxic/metabolic diseases, and genetic diseases. Neurons undergoing acute cell death often show red (eosinophilic) cytoplasm and pyknotic nuclei histologically and are referred to as red neurons (Figure 21-2). Neurons may also undergo programmed cell death (apoptosis). Central chromatolysis refers to the changes that occur in the neuronal cell body (usually a lower motor neuron) when its axon is injured (axonal reaction). This axonal reaction is characterized by enlargement of the neuronal cell body, displacement of the neuron’s nucleus to the periphery of the cell body, and disappearance of the more centrally located Nissl bodies (stacks of rough endoplasmic reticulum). The more peripherally located Nissl bodies remain, hence the term central chromatolysis.
Astrocytes are a type of glial cell that provide numerous support functions for neurons. Astrocytes are somewhat analogous to fibroblasts elsewhere in the body, in that astrocytes react to injury by forming glial filaments (scar tissue). In contrast to the extracellular collagen fibers produced by fibroblasts, glial filaments are intracytoplasmic. The glial filaments immunostain for glial fibrillary acidic protein (GFAP) and highlight the star-shaped astrocytes (Figure 21-3). Gliosis (reactive astrocytosis)—the reaction of astrocytes to a brain injury—occurs in many pathologic contexts (e.g., ischemia, trauma, infection, neurodegenerative diseases, demyelinating diseases). Normally, the cytoplasm of an astrocyte is inconspicuous, so that the astrocytic nuclei appear to have no cytoplasm. However, after a brain injury, astrocytes proliferate and develop large amounts of perinuclear cytoplasm as they synthesize glial filaments. These reactive astrocytes are known as hypertrophic or “gemistocytic” astrocytes. After an injury, reactive astrocytosis becomes evident histologically after 4 days, well established after 7–10 days, and maximal at 2–3 weeks. Alzheimer type II glia are astrocytes with enlarged, pale nuclei, and inconspicuous cytoplasm that develop during hyperammonemic states, such as those associated with acute liver failure or a portosystemic shunt secondary to hepatic cirrhosis. This “metabolic astrocytosis” reflects the major role of astrocytes in the detoxification of ammonium ion in the CNS.
Oligodendrocytes (Figure 21-3) are a type of glial cell that myelinate axons in the CNS. Direct injury of the myelin sheath or its oligodendrocyte may result in breakdown of the myelin sheath. If the myelin breakdown is associated with preservation of the underlying axon, the process is referred to as demyelination. Degeneration of the underlying axon always leads to breakdown of the axon’s myelin sheath.
Ependymal cells are a type of glial cell that line the ventricles of the brain (Figure 21-4) and the central canal of the spinal cord. Unlike astrocytes, ependymal cells do not proliferate in response to a brain injury.
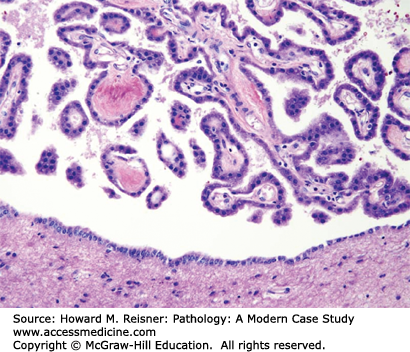
The epithelial cells of the choroid plexus closely resemble ependymal cells and cover the fibrovascular papillae of the choroid plexus (Figure 21-5). Choroid-plexus tissue is found in the lateral, third, and fourth ventricles and produces the cerebrospinal fluid (CSF).
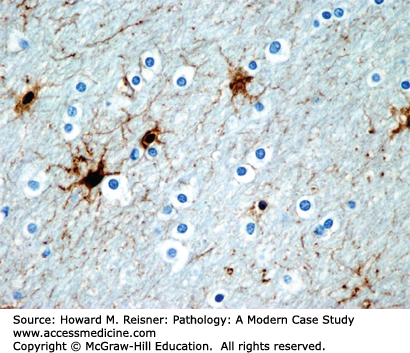
Microglia (Figure 21-6) are phagocytic cells and function as the macrophages of the CNS. The brain contains several different macrophage/monocyte populations, including resident brain macrophages (microglia) and perivascular macrophages. All of these macrophage/monocyte cells are of bone marrow origin and have roles in phagocytosis of debris and in immune surveillance. The resident microglia handle the cleanup of minor debris, but the cleanup of larger amounts of necrotic debris requires the recruitment of blood-borne monocytes/macrophages to the area. Activated macrophages within the brain typically have spindle-shaped nuclei (rod cells). As the macrophages ingest debris, they become the lipid-filled macrophages (foam cells) often found around areas of brain necrosis. Small clusters of microglial cells within the brain parenchyma (microglial nodules) are a distinctive, but not pathognomonic, feature of viral and rickettsial encephalitis. The multinucleated giant cells that characterize HIV-associated encephalitis are also of macrophage origin.
Intracranial pressure (ICP) is the pressure inside the cranium and is normally 1–15 mmHg. The skull serves as a rigid vault enclosing a fixed volume of brain, blood, and CSF. Any increase in volume of one component means that one or both of the other components must be reduced in volume (Monro–Kellie hypothesis). Cerebral perfusion pressure (CPP) is the mean arterial pressure minus the mean ICP. Thus, as the ICP increases, the CPP decreases. There are multiple causes of increased ICP (Table 21-1), with mass lesions being the most common cause. Increased ICP is evidenced clinically by headache, nausea, vomiting, and papilledema (swelling of the optic disc). The elevated ICP also causes enlargement of the head and a tense or bulging anterior fontanel in young children in whom the cranial sutures have not yet fused.
|
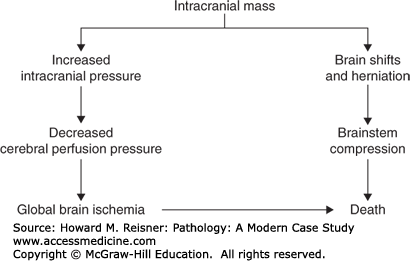
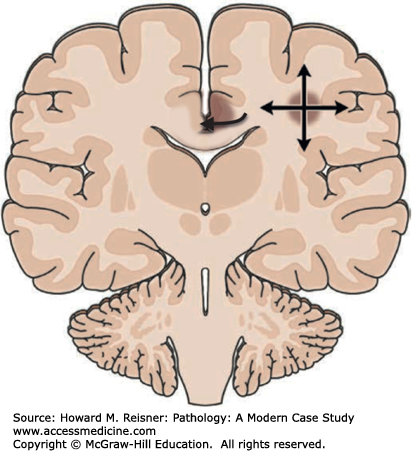
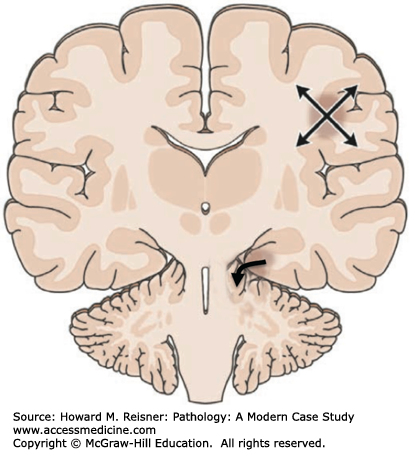
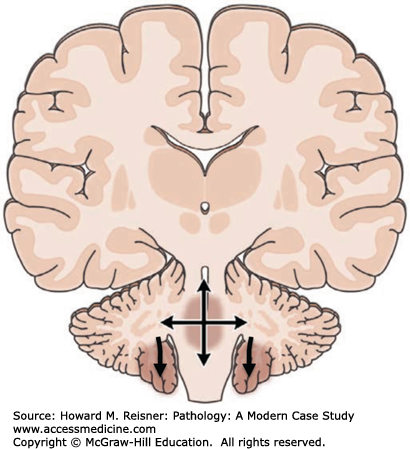
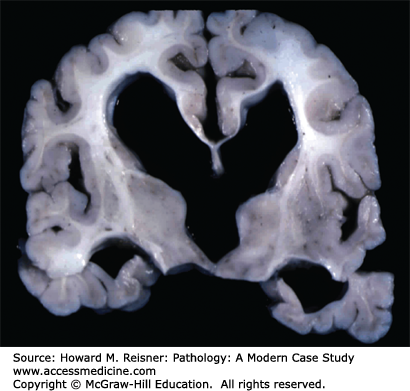
Major complications of a mass lesion and raised ICP are brain ischemia and brain herniation (Figure 21-7). Brain ischemia occurs when the raised ICP approaches systemic arterial blood pressure, thereby causing decreased cerebral perfusion and brain ischemia. Brain herniation occurs when there is a displacement or shift of brain tissue from one intracranial compartment to another. The major forms of brain herniation are subfalcine, transtentorial, and tonsillar. A subfalcine herniation (Figure 21-8) occurs when the cingulate gyrus herniates horizontally under the falx cerebri. Transtentorial (uncal, central diencephalic) herniation (Figure 21-9) occurs when the medial aspect (uncus) of one temporal lobe or the lower diencephalon herniates downward through the tentorial incisura. The herniating uncus compresses the ipsilateral third cranial nerve and midbrain, leading to dilatation of the ipsilateral pupil, contralateral hemiparesis, impairment of upper brain stem functions, and midbrain hemorrhages. Tonsillar herniation (Figure 21-10) occurs when the cerebellar tonsils herniate downward through the foramen magnum because of a mass in the posterior fossa. The herniating tonsils compress the medulla, causing apnea and death.
The term edema refers to an abnormal accumulation of fluid in tissue spaces. Brain edema accompanies many disease processes. The edema causes brain swelling and magnifies any mass effect caused by the underlying pathologic process. Brain edema thus contributes to an increase in the ICP and to the possibility of a brain herniation. Brain edema is classified as vasogenic, cytotoxic, or interstitial.
Vasogenic brain edema—extracellular edema due to increased permeability of the blood–brain barrier (BBB). The BBB is an anatomical and physiologic barrier that helps maintain a controlled extracellular environment around the neurons and glial cells of the CNS. The barrier is located at the level of the brain capillaries and regulates the movement of molecules into the extracellular space of the brain. The BBB helps prevent the entry of blood-borne infectious agents and toxins into the brain but also may impede the delivery of antibiotics and chemotherapeutic agents to the CNS. Many disease processes are associated with breakdown of the BBB. The increased capillary permeability leads to greater passage of osmotic particles into the brain’s extracellular space and results in an extracellular accumulation of water (vasogenic brain edema). Vasogenic edema is found primarily in the white matter. The integrity of the BBB is a useful marker of disease and can be assessed radiologically by administering an intravenous contrast agent prior to the radiologic study. When the BBB has been compromised by a pathologic process, the contrast agent will leak out of the brain capillaries into surrounding brain tissue and can be seen in the radiologic images.
Cytotoxic (cellular) brain edema—intracellular edema (hydropic cellular swelling) due to an osmotic imbalance between the cell and the extracellular fluid. There is no expansion of the extracellular space. The cell may swell because of loss of control of ion movements across its cell membrane (e.g., with cerebral ischemia, the cell does not have sufficient energy for its ion pumps) or because of an increased imbalance between intracellular and extracellular osmolarity (e.g., acute water intoxication). Cellular edema is found primarily in the gray matter and is most commonly associated with acute ischemic brain injury. Radiographically, cytotoxic edema is not associated with a positive contrast study, since there is no breakdown of the BBB.
Interstitial (hydrocephalic) brain edema—extracellular edema arising from the transependymal flow of CSF. The transependymal movement of CSF is due to increased CSF pressure, which is caused by an obstruction of CSF pathways. The obstruction of normal CSF flow also causes ventricular dilatation (hydrocephalus). The transependymal edema is in the periventricular white matter surrounding the dilated ventricles and can be seen radiologically.
Combined forms of brain edema are common. For example, the brain edema associated with ischemia is a combination of cytotoxic edema and vasogenic edema. The cytotoxic edema develops almost immediately after the onset of the brain ischemia, while the vasogenic edema develops hours to days later.
Hydrocephalus literally means increased water (CSF) in the head. Hydrocephalus is traditionally defined as enlarged ventricles (ventriculomegaly) due to the obstruction of the bulk flow of CSF (Figure 21-11). Normally, the total volume of CSF is around 150 mL, of which about 25 mL are within ventricular system and the remainder in the craniospinal subarachnoid space. Approximately 500 mL of CSF are produced each day. The CSF has multiple functions, including providing buoyancy for the brain, maintaining a highly regulated extracellular fluid compartment for the cells of the brain and spinal cord, and providing a buffer so that changes in the volume of the other intracranial components (i.e., brain and blood) can occur within the intracranial cavity.
Hydrocephalus may be classified as noncommunicating hydrocephalus if the obstruction of CSF flow is within the ventricular system (e.g., stenosis of the cerebral aqueduct between the third and fourth ventricles) or as communicating hydrocephalus if the obstruction is within the subarachnoid space or arachnoid villi (e.g., complication of subarachnoid hemorrhage, SAH). Hydrocephalus may be acute or chronic in onset and may be present at birth (congenital hydrocephalus) or acquired later in life.
Obstruction of CSF flow in adults leads to hydrocephalus and clinically to signs and symptoms of increased ICP—headache, nausea, vomiting, and papilledema. In young children, who have an expandable cranium because their sutures have not yet fused, the obstruction of CSF flow leads to hydrocephalus and a progressively increasing head circumference. In both adults and children, the ventricles enlarge at the expense of the periventricular white matter, so that hydrocephalus can produce considerable neurologic deficits over time.
CASE PRESENTATION: Mass Lesion
A 40-year-old woman with a history of HTN presents to the local ED with symptoms of nausea, vomiting, vertigo, weakness in the legs and a headache localized to the “back of her head” progressing over the past 24 hrs. An MRI was performed (left panel) (Figure 21-12).
MRI discloses an acute left-sided cerebellar infarct with tonsillar herniation, obstructive hydrocephalus, and cerebral edema. (Right panel) Medical illustration, worth 1000 words.
In this case, an emergency resection of involved cerebellum with placement of a shunt was a life-saving measure.
HUNTING FOR ZEBRAS
Curiously, ventriculomegaly develops in some adults who have no obvious increase in CSF pressure and no demonstrable obstruction of CSF flow. These patients are thought to have a chronic communicating hydrocephalus due to periodic increases in the ICP. This syndrome, termed normal pressure hydrocephalus (NPH), is evidenced clinically by the triad of dementia, gait ataxia, and urinary incontinence. Placement of a CSF shunt may be beneficial in patients with NPH.
Ventricular enlargement may also be a consequence of brain atrophy. Ventriculomegaly secondary to brain atrophy (hydrocephalus ex vacuo) is not related to a blockage of CSF flow, is not associated with increased ICP, and does not benefit from placement of a CSF shunt.
There are many causes of hydrocephalus (Table 21-2). In infants and young children, hydrocephalus is often due to a congenital malformation, such as a congenitally malformed cerebral aqueduct. Inflammation, hemorrhage, or neoplasm within the ventricular system or subarachnoid space may cause obstruction of CSF flow and hydrocephalus in both children and adults.
|
INFECTIONS
The nervous system is susceptible to many infectious agents, including bacteria, viruses, fungi, amebae, and parasites. CNS infections are oftentimes life threatening with serious consequences. It is convenient to divide infections into (1) infections of the leptomeninges (pia mater and arachnoid mater) and associated CSF (meningitis), (2) infections of brain parenchyma (encephalitis), and (3) localized collections of pus within the brain parenchyma (brain abscess).
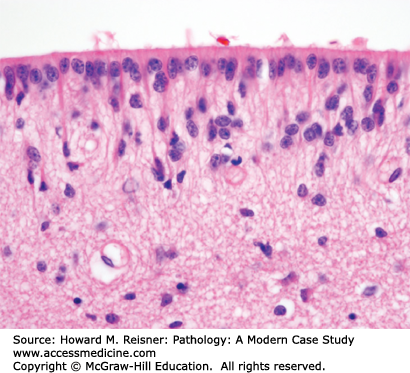
Acute bacterial meningitis is an acute inflammation (neutrophils) of the leptomeninges due to bacteria growing within the CSF. Clinical features of headache, fever, stiff neck, and altered mental status have their onset over hours to days. The bacteria often reach the CSF through the bloodstream. Sometimes, the bacterial infection spreads to the meninges from a parameningeal infection in the sinuses or skull. Head trauma, especially penetrating injuries, and neurosurgical procedures, such as a ventriculoperitoneal shunt, are also potential causes of infection. Age is an important factor in determining the type of bacterial infection in community-acquired meningitis (Table 21-3) Hospital-acquired (nosocomial) meningitis is most often due to gram-negative rods. Clinicopathologic correlates of acute bacterial meningitis include stiff neck and headache from meningeal inflammation and mental status changes and seizures from toxins in the pus within the CSF. These toxins are also responsible for brain edema and its associated mass effect. The acute inflammatory process may involve blood vessels and cranial nerves in the subarachnoid space, resulting in brain infarcts and cranial nerve palsies. The pus or postinflammatory meningeal fibrosis may also obstruct CSF flow and lead to a communicating hydrocephalus.
Chronic meningitis may complicate infections characterized by chronic or granulomatous inflammation. Clinical features of chronic meningitis include headache, fever, stiff neck, and altered mental status. Although purulent meningitis has an acute (hours to days) clinical course, chronic meningitis has a subacute (weeks) to chronic (months) clinical course. The chronic or granulomatous inflammatory infiltrates preferentially involve the leptomeninges at the base of the brain and the associated structures (blood vessels and cranial nerves) in the basal subarachnoid space. Inflammation of blood vessels leads to thrombosis and consequent brain infarcts; inflammation of cranial nerves leads to cranial nerve palsies. The chronic inflammatory process also causes meningeal fibrosis, which may obstruct CSF flow within the subarachnoid space and produce a communicating hydrocephalus.
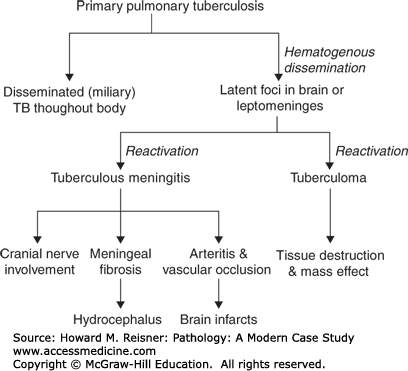
Diseases associated with chronic meningitis include tuberculosis, syphilis, some fungal infections (e.g., cryptococcosis, coccidioidomycosis, blastomycosis), and Lyme disease (Borrelia burgdorferi). Sarcoidosis, while not an infectious disease, also causes chronic granulomatous meningitis. The pathogenesis of CNS tuberculosis is illustrated diagrammatically in Figure 21-13. CNS complications of longstanding syphilis include chronic leptomeningitis and obliterative arteritis (meningovascular syphilis), chronic encephalitis with neuronal loss (paretic dementia), and chronic inflammation of the dorsal roots and ganglia with loss of dorsal-root ganglion cells and degeneration of the posterior columns of the spinal cord (tabes dorsalis).
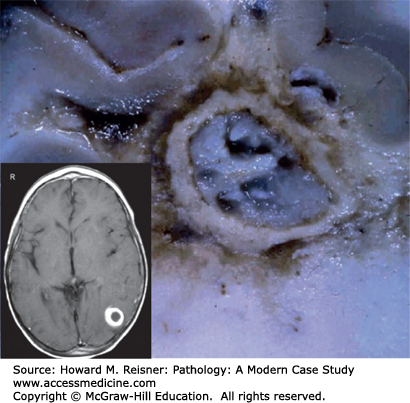
A brain abscess is a localized collection of pus within the brain parenchyma (Figure 21-14). The pus is walled off from the surrounding brain tissue by a layer of highly vascularized fibrous tissue (granulation tissue). The surrounding brain is edematous and has a reactive gliosis. As the abscess enlarges, it usually tracks centripetally through the white matter toward the ventricular system. This centripetal spread is thought to be due to the white matter having relatively less vascularity than the gray matter.
The pathogenesis of a brain abscess often involves bacteremia from a distant focus of infection. Bacteremia-associated brain abscesses are frequently multiple and often begin at the junction between cortex and white matter. Direct extension of a parameningeal infection (e.g., sinusitis, mastoiditis) is another cause of brain abscess. Penetrating head trauma is a potential, but uncommon, cause of brain abscess. Brain abscesses often contain a mix of aerobic and anaerobic bacteria; less commonly, fungi are responsible.
A brain abscess causes tissue destruction, with resultant neurologic defects, and mass effect, with increased ICP and risk of herniation. An abscess may rupture into the ventricular system, causing ventriculitis and usually leading to death.
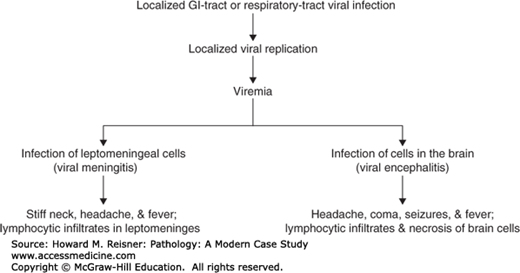
Viral infections of the CNS may cause a meningitis or encephalitis (Figure 21-15). If the virus selectively infects cells of the leptomeninges, the inflamed meninges (meningitis) are associated with headache and a stiff neck. Viral meningitis is characterized histologically by lymphocytic infiltrates in the leptomeninges. Enteroviruses are the most common cause of viral meningitis. Other common causes include arboviruses, HIV, HSV-2, mumps virus, and lymphocytic choriomeningitis virus. The infectious agent is often not cultured in cases of lymphocytic meningitis, so that the disease is sometimes referred to as an “aseptic” meningitis. Viral meningitis is usually a benign, self-limited disease.
In viral encephalitis, the virus selectively infects cells of the brain (Figure 21-15). The inflamed brain (encephalitis) is associated with an altered mental state, neurologic dysfunction, and often seizures. Viral encephalitis is characterized histologically by microglial nodules and perivascular lymphocytic infiltrates within the brain tissue and variable amounts of brain necrosis and hemorrhage (Figure 21-16). Common causes of viral encephalitis include HSV-1, Varicella–Zoster virus, arboviruses (e.g., Eastern & Western equine encephalitis), enteroviruses (including poliovirus), measles virus, and mumps virus. Some viruses are associated with intranuclear [e.g., HSV-1, progressive multifocal leukoencephalopathy (PML), CMV] or intracytoplasmic (e.g., CMV, rabies) viral inclusions. Some viruses typically show a characteristic involvement of certain cell types or areas of the CNS (Table 21-4).
|
FIGURE 21-16
HSV-1 encephalitis is the most commonly occurring sporadic viral encephalitis. This young adult presented with confusion and later developed seizures and coma and died. The horizontally cut postmortem brain specimen shows the characteristic predilection of HSV-1 for the temporal lobe. Note that HSV-1 encephalitis is very destructive, causing necrosis and hemorrhage in the brain. Microscopically, the brain necrosis is accompanied by perivascular lymphocytic infiltrates, microglial nodules, and intranuclear viral inclusions. Viral leptomeningitis, in contrast, does not show inflammation and necrosis of brain tissue and has a benign clinical course.
The pathogenesis of viral encephalitis involves viral entry into the CNS via two main routes—bloodstream or axons. Many viruses use a viremia to gain access to the CNS. These hematogenously disseminated viral infections, which include the enteroviruses and arboviruses, first proliferate locally in the respiratory tract, GI tract, or skin, then disseminate hematogenously (viremia) to the CNS.
Rabies virus and herpesviruses gain entry to the CNS via axons. In the case of rabies virus, the virus first proliferates locally in the region of the wound (bite), and then travels centripetally along peripheral nerves via retrograde axonal transport to the spinal cord and brain.
HIV encephalitis is the result of direct infection of the CNS by HIV, causing a subacute encephalitis with multinucleated giant cells. The subacute encephalitis is characterized by perivascular chronic inflammation, foci of reactive gliosis, and scattered microglial nodules. Also present and virtually pathognomonic are multinucleated giant cells, which are derived from macrophages and contain HIV antigens. There may also be areas of pallor and gliosis of the cerebral white matter (HIV leukoencephalopathy). In infants and children, HIV encephalitis may also be accompanied by calcification of small cerebral blood vessels.
Fungal infections account for an increasing proportion of CNS infections due to the widespread use of immunosuppressive therapy, the increase in the relative numbers of aging individuals, and AIDS. Common clinical presentations are a chronic granulomatous meningitis, brain abscess, or fungal arteritis with brain infarcts. Cryptococcosis, blastomycosis, coccidioidomycosis, and histoplasmosis most often present as a chronic meningitis. Aspergillosis often presents as a fungal arteritis with brain infarcts.