NORMAL LUNG ANATOMY AND PHYSIOLOGY
Normal adult lung is a sophisticated system of conducting airways and gas exchange surfaces. The foregut develops a ventral outpouching that progressively bifurcates to create branch points (“carinas”) for conducting airways (bronchi, then bronchioles, then alveolar ducts). Conducting airways form during the “pseudoglandular” phase by 15 weeks’ gestational age, and proto-alveoli are evident in loose mesenchyme during the “canalicular” phase by 24 weeks’ gestational age. The mesenchyme is progressively excluded or flattened between developing alveoli, such that an adult lung has back-to-back alveolar airspaces separated by elastin-rich, capillary-rich interstitial stroma.
In parallel with the development of the functional adult lung structure, the critical type II epithelial cell matures and begins to excrete surfactant, a natural detergent that breaks the water tension of the thin layer of water that covers the alveolar wall. Without type II cell surfactant, the newborn cannot generate enough mechanical force to inflate the alveoli, and will rapidly fatigue and arrest. The probability of surfactant deficiency varies inversely with fetal age, such that surfactant deficiency will be seen in >50% of fetuses <28 weeks’ gestational age. The fetal age at which 95% of fetuses can ventilate normally after delivery is 36 weeks. Fetuses delivered before that age are monitored aggressively and treated promptly with synthetic surfactant if they have persistent atelectasis (collapse of lung tissue) and begin to tire.
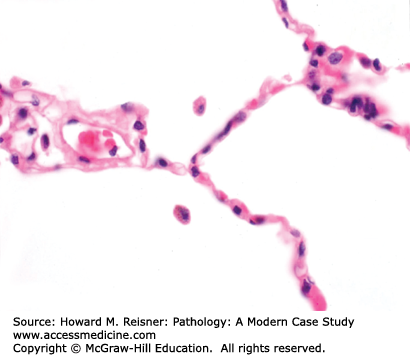
The anatomy of the adult lung is best described starting at the larynx and proceeding toward the alveoli. The trachea and bronchi are encircled by near-circumferential cartilage rings that prevent collapse of these large-caliber airways during the expiratory phase of the ventilatory cycle. These airways show lush surface cilia and submucosal glands that excrete mucin. Moving distally in the conducting airways, the cartilage rings and submucosal glands are lost; these small-caliber airways are called bronchioles. Like the trachea and bronchi, bronchioles have lush surface cilia. The bronchi and bronchioles run adjacent to pulmonary artery branches of similar caliber. Alveolar ducts lose the cilia, as one enters the grapelike clusters of alveoli, the critically important sites for countercurrent exchange of inhaled O2 and RBC-bound CO2. Incomplete ridges subdivide this surface area into intersecting spheres that in tissue sections look like incomplete circles separated by thin, delicate, capillary-rich alveolar septae. The alveoli are lined by a watertight monolayer of type I cells. Efficient gas exchange requires minimal diffusion distance, and evolution has left us with a fused basement membrane in common between the alveolar surface type I epithelial cells and the underlying capillary endothelial cells. The capillaries within the alveolar septae are surrounded by abundant elastin, and together comprise the interstitium (Figure 8-1).
QUICK REVIEW Adult Lung Physiology
Ventilation volumes, flow rates, ventilation:perfusion (V:Q) matching, and gas exchange are important to the pulmonologist, whereas structure:function correlation and three chemical components (surfactant, elastin, and mucin) of the pulmonary system are important to the pathologist. The normal respiratory tree has unobstructed conducting airways, clean/dry alveoli, and thin delicate alveolar septae. Upon ventilation and perfusion of these alveoli, gas exchange occurs. Surfactant allows water tension in the alveoli to be broken. Elastin provides elastic recoil to keep lungs from overinflating. Mucin traps inspired dust before it reaches the alveoli.
Lungs could not inflate unless type II epithelial cells make surfactant that serves as a detergent. Without surfactant, the surface tension of the water layer in the 3 × 108 alveoli comprising 80 m2 of alveolar surface could not be overcome to allow inflation of the adult lung, and certainly that of the newborn lung.
The lung, removed from the chest, would collapse because of the large amount of elastin in its interstitium. In vivo, vacuum pressure in the pleural space maintains the inflated state of the normal lung. If you lacerate the parietal pleura with a knife, the vacuum will be broken, and the lung will collapse. When present, elastin allows effortless expiration during the ventilatory cycle. When elastin is absent, alveoli overinflate and rupture, leading to increased expiratory work, increased endexpiratory volumes, and histologic changes called emphysema (discussed later in the chapter). Therefore, normal ventilation requires surfactant to break the surface tension of water in the alveoli, a vacuum in the pleural space, and sufficient mechanical force by the chest wall and diaphragm to draw air into the alveoli.
Bronchial submucosal glands excrete mucin into ducts that lead to the ciliated surface of the airway. This mucin acts to catch inhaled dust and debris before it can reach the clean and dry alveoli. Surface cilia are powered by ATP to beat in a coordinated unidirectional fashion, sweeping the normally thin mucin layer with its dust and debris up/proximally/centrally, so that the mucin can be swallowed or coughed out. The net result is that the alveoli are protected from receiving or accumulating a burden of dust, debris, or mucin that would interfere with gas exchange.
Countercurrent diffusion between alveolar air O2 and capillary RBC hemoglobin CO2 is referred to as gas exchange, and occurs across two attenuated cell types (type I epithelium and endothelium) and a shared/fused basement membrane. O2 and CO2 diffuse according to their relative concentration gradients, unique diffusion characteristics for each molecule, and the diffusion distance, according to Fick’s law. Anything that interferes with ventilation (e.g., blood, pus, pond water, or fibrin), small molecule diffusion (e.g., septal fibrosis), or capillary blood flow (e.g., thromboemboli and heart failure) interferes with this gas exchange, and can lead to V:Q mismatch. Mismatch in which the lung is perfused but not ventilated is called shunting. Mismatch in which the lung is ventilated but not perfused is called dead space. Ideally, there is physiologic autoregulation of V:Q. V:Q mismatch can be measured by the pulmonologist.
CASE 8-1
Clinical: A 65-year-old man with 30 pack-year smoking history, with new bloody sputum.
Radiology: Posteroanterior (PA) chest X-ray shows a solitary right upper lobe mass. CT scan shows the lung mass and hilar lymphadenopathy. Superimposed PET scan showed FDG signal in the lung mass and in the nodes.
Course: A transbronchial biopsy was performed.
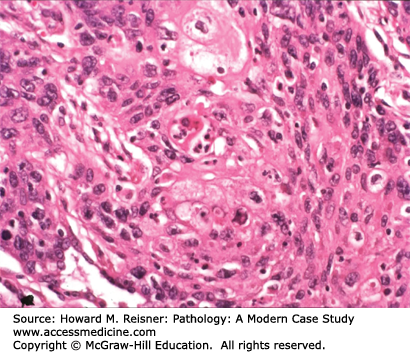
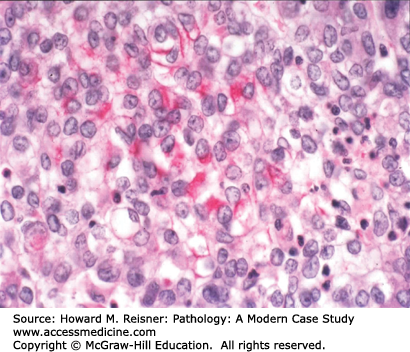
Pathology: Hematoxylin and eosin (H&E)-stained section shows an invasive malignant neoplasm with cell–cell cohesion, focal desmosome formation, and focal cytoplasmic keratin accumulation (Figure 8-2). These features support squamous differentiation. The final diagnosis is invasive squamous cell carcinoma. Metastasis should be excluded. A computer search for previous biopsy material from this patient would be performed to formally exclude primary squamous carcinoma in another organ (e.g., head/neck mucosa or uterine cervix (if a female patient)) that could have metastasized to the lung.
LUNG NEOPLASMS
Most neoplasms in the lung are malignant neoplasms of epithelial derivation, that is, carcinomas. Almost all (>85%) lung carcinomas are causally associated with cigarette smoking. We know this because of the above association between lung carcinoma incidence and smoking, but more importantly because the relative risk of lung carcinomas increases with cigarette smoking exposure, and decreases following smoking cessation.
What are the clinical implications of cigarette smoking as it relates to lung carcinomas? Roughly 200,000 new cases of lung carcinoma are expected in the United States next year. Hence, a legal, commercial product resulted in about 170,000 [(200,000)(0.85)] new patients with a potentially lethal malignancy. Given the 20% average 5-year survival rate for lung carcinoma, 136,000 of these newly diagnosed smokers with lung carcinoma will die within the next 5 years, possibly unnecessarily, in spite of best clinical efforts to manage their disease. With the adoption of smoking by women, lung carcinoma incidence and mortality rates for women have risen proportionately over the last 30 years, such that lung carcinomas are now the most common cause of death from neoplasm in women the United States. Few realize that lung carcinomas kill 50% more women each year than breast carcinoma. These are striking examples of unnecessary morbidity and mortality in America resulting from etiologic agents that are components of a commercial product. As an aside, if tobacco were made as expensive as designer drugs, consumption would decrease, and lung carcinomas, head/neck squamous carcinoma, chronic bronchitis, and emphysema would likely become orphan diseases in the United States.
Primary lung carcinoma is typically a unifocal clonal proliferation, recognizable microscopically as one of a limited number of types. Most primary lung carcinomas grow as dominant masses within the lung, and can be recognized with noninvasive radiographic techniques. Carcinoma can obstruct a large conducting airway, with development of either sterile or infectious pneumonias distal to the airway obstruction. Invasive carcinoma can erode into pulmonary arterial branches, and can result in exsanguination. Most commonly, carcinomas cause morbidity and mortality by metastasizing via blood vessels to other organs (e.g., brain, bone, liver, adrenal, and even skin). It is usually the disruption of normal function of other organs late in the course of the disease, as well as accompanying pain and cachexia, that lead to death.
WHAT WE DO
Lung carcinomas are a heterogeneous group of neoplasms that the pathologist can distinguish morphologically by identifiable features of squamous carcinoma (desmosomes and/or keratin production), adenocarcinoma (glandular/papillary architecture +/– mucin production), or neuroendocrine neoplasms (coarse chromatin, neuroendocrine protein expression). Well- and moderately differentiated carcinomas are those that manifest recognizable features that allow subcategorization into squamous cell carcinoma, adenocarcinoma, or neuroendocrine neoplasms. Poorly differentiated or undifferentiated carcinomas may result in a differential diagnosis that includes not only carcinoma but also melanoma and lymphoma, requiring a panel of immunostains and mucin stains to clarify lineage and subtype. Because of major differences in clinical presentation and clinical therapies, the main clinical categories are “non-small cell carcinomas” (including squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, large cell undifferentiated carcinoma (LCUC), sarcomatoid carcinoma, carcinoid tumors, salivary gland type carcinomas) and “small cell carcinoma.” Descriptions of these different neoplasms follow.
Squamous cell carcinoma of the lung is a disease of cigarette smokers, people breathing second-hand smoke, uranium miners, and rare patients with laryngeal human papillomavirus (HPV) infection. Roughly 20% of US adults (both men and women) smoke, whereas there are very few uranium miners, so most lung squamous cell carcinoma patients have a smoking history. Primary lung squamous cell carcinomas account for roughly 35% of all new lung carcinoma cases.
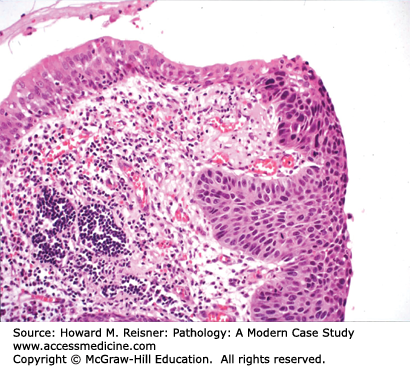
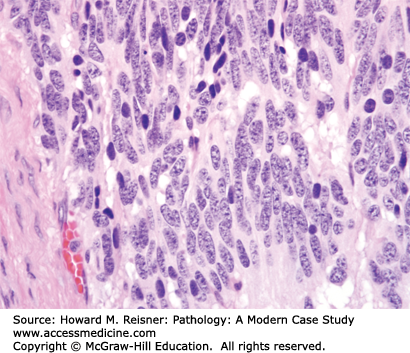
Most squamous cell carcinomas of lung develop in the bronchi, hence the old name, “bronchogenic” (born in the bronchus) carcinoma. Radiographically, these bronchial primaries are central in location. Squamous cell carcinoma develops from preexisting squamous cell carcinoma in situ, that is, full-thickness dysplasia developing in a background of squamous metaplasia (Figure 8-3). The presumed pathogenesis is that hot gases and entrained irritants in inhaled cigarette smoke cause the normal ciliated respiratory epithelium to change to squamous mucosa (squamous metaplasia). Continued exposure to entrained organic chemicals, particularly polyaromatic hydrocarbons, leads to the accumulation of mutations and DNA copy number changes, reflected in morphologic features of dysplasia (architectural disarray, nuclear pleomorphism, nuclear membrane irregularities, nuclear hyperchromasia, and increased mitotic rate). Squamous cell carcinoma confined to the surface epithelium and delimited by the basement membrane (squamous cell carcinoma in situ) has no metastatic potential, because it does not have access to either lymphatics or blood vessels deep to the basement membrane. However, once the malignant clone invades across the basement membrane (invasive squamous cell carcinoma) (Figure 8-2), it now has the potential to invade into lymph or blood vessels, and to metastasize to regional nodes (via lymphatics) or to distant organs (via systemic blood flow). Well- to moderately differentiated invasive squamous cell carcinoma is identified by virtue of desmosome formation (intercellular bridges) and cytoplasmic accumulation of keratin [cytokeratin (CK)], an intermediate filament protein. At the protein level, over 95% of squamous cell carcinomas express CK 5/6 and P63, whereas few express thyroid transcription factor-1 (TTF-1) or aspartic protease napsin A, each of which can be detected by immunohistochemical (antibody-based) stains. At the molecular level, most squamous cell carcinomas show P53 mutations and disruption of the RB pathway, but fewer KRAS mutations than are seen in adenocarcinomas.
The prognosis of squamous cell carcinoma, like other common NSCLC, is predicted based on stage (extent of disease, including size of the primary (T), extent of local invasion, presence of regional nodal metastases (N), and presence of distant metastasis (M)), performance status, and weight loss history.
Adenocarcinoma of the lung is a disease of both smokers and nonsmokers. If you are a lifelong nonsmoker with a new primary lung carcinoma, adenocarcinoma is the most likely diagnosis. Roughly 30% of new lung carcinomas will be diagnosed as adenocarcinomas.
Most adenocarcinomas present as distal/peripheral lung masses, in contrast to the central location of most squamous cell carcinomas. At the histologic level, there is an early group of adenocarcinomas that are limited to surface epithelial involvement. These have traditionally been called bronchioloalveolar carcinomas (BAC). BAC can show either mucinous or nonmucinous differentiation. Neoplasms with this architecture that are <3 cm in size are now considered to be adenocarcinoma in situ (BAC/ACIS), with expected 5-year survival of 100% following negative-margin resection. Tumors <3 cm in size that are associated with invasion across the basement membrane, with resulting stromal response and remodeling of lung parenchyma, are considered minimally invasive adenocarcinomas if the invasive component is <0.5 cm. Tumors are considered invasive adenocarcinomas if the invasive component is >0.5 cm or the total tumor diameter is >3 cm.
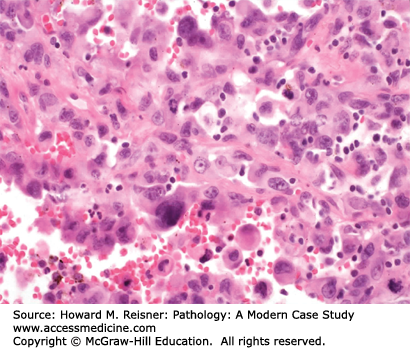
Large cell undifferentiated carcinoma (LCUC) of the lung is the least common of the major types of NSCLC, comprising roughly 10% of patients with new lung carcinomas. These carcinomas can develop peripherally or centrally. The cell of origin for LCUC is unclear. At the histologic level, carcinoma cells are undifferentiated, with large nucleoli, more cytoplasm than expected for small cell carcinoma, and without any of the identifiable features of squamous cell carcinoma or adenocarcinoma (Figure 8-6). Molecular studies suggest that LCUC is a distinct disease entity, rather than just an undifferentiated form of more easily recognized NSCLC.
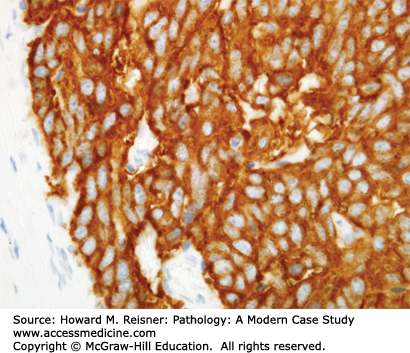
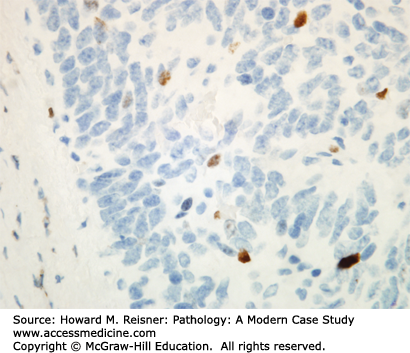
Other Non-Small Cell Lung Cancer: The remaining 5% of new lung tumor diagnoses are a potpourri of unusual neoplasms. Carcinomas that show prominent spindle cell or giant cell component are called “sarcomatoid” carcinomas. Carcinomas that mimic salivary gland carcinomas (adenoid cystic carcinoma, mucoepidermoid carcinoma) can arise from submucosal glands in the central tracheobronchial submucosa. Finally, there are three neuroendocrine neoplasms that are not small cell carcinomas (small cell carcinoma is discussed separately below). Mature carcinoid tumor typically presents in a young adult as a nested submucosal mass with airway obstruction, but is unlikely to metastasize, so can be treated with surgical resection alone. It is recognizably neuroendocrine, with nested growth, coarse chromatin (Figure 8-7), and with immunoreactivity for chromogranin and synaptophysin (Figure 8-8). However, it does not have prominent nucleoli or coagulative tumor cell necrosis, and it shows low mitotic activity and low growth fraction (as detected with Ki-67 antibody (Figure 8-9)). Carcinoid tumors with nucleolar prominence, focal mitotic activity, and focal coagulative tumor cell necrosis are called atypical carcinoid tumors. These have increased risk of metastasis, so may be considered for adjuvant chemotherapy following resection. At the high grade end of the neuroendocrine neoplasm spectrum is large cell neuroendocrine carcinoma, recognizable as a neuroendocrine carcinoma, with similar features (high mitotic rate, frequent apoptosis, frequent coagulative necrosis) to small cell carcinoma, but with significant cytoplasm and prominent nucleoli (unlike small cell carcinoma).
IN TRANSLATION
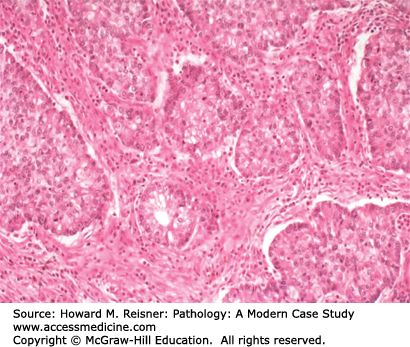
Invasive well- to moderately differentiated adenocarcinomas can be diagnosed by virtue of papillary, glandular/acinar (Figure 8-4), or solid architecture, as well as by mucin production (Figure 8-5). At the protein level, roughly 75% of adenocarcinomas express TTF-1 and napsin A, and few express CK5/6 or P63 by immunohistochemical stains. At the cytogenetic and molecular level, most lung adenocarcinomas have some combination of mutations (EGFR, KRAS, BRAF, and P53), and/or fusion gene formation (EML4/ALK inversion translocation). The recent availability of small molecule inhibitors for mutant gene products of EGFR, BRAF, and ALK has made screening for these mutations an important aspect of case workup.
CASE 8-2
Clinical: An 80-year-old man with 50 pack-year smoking history, with new bloody sputum.
Radiology: PA chest X-ray shows a hilar and mediastinal mass. CT scan shows the central lung mass with associated hilar/mediastinal lymphadenopathy.
Course: A transbronchial biopsy was performed.
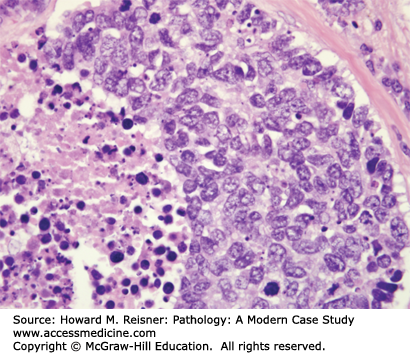
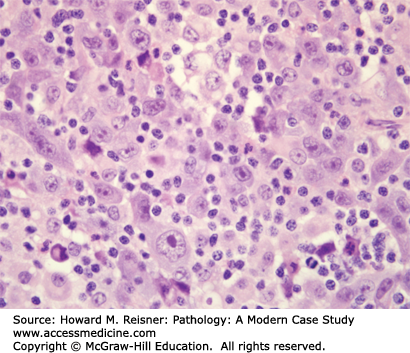
Pathology: H&E-stained section shows an invasive malignant neoplasm with hyperchromatic nuclei, coarse “salt-and-pepper” chromatin, absent nucleoli, high N:C ratios, frequent mitotic figures, and coagulative necrosis (Figure 8-10). These features support neuroendocrine differentiation in a high-grade malignant neoplasm. The final diagnosis is small cell lung carcinoma (SCLC). Staging should be performed, but the expected stage is late/high, and the expected prognosis is poor.
Small cell carcinoma deserves a separate category within the lung carcinoma classification, based on its unique clinical presentation and differences in clinical management. Small cell carcinoma is unique because it typically metastasizes early, thereby presenting as late-stage disease in the majority of cases. Patients presenting with late-stage disease are treated with systemic chemotherapy to kill clinical/subclinical metastases and the primary, as well as radiotherapy for additional local control of the primary. Small cell carcinoma accounts for roughly 20% of newly diagnosed cases of lung carcinoma. It has some features in common with squamous cell carcinoma, that is, smoking history and central location of the primary. A subset of these carcinomas generates bioactive hormones, such that an SCLC patient might present with Cushing syndrome secondary to ectopic ACTH production. Like squamous cell carcinoma and adenocarcinoma, metastases can traffic via lymphatics to regional lymph nodes, and can traffic to distant sites via the systemic bloodstream.
IN TRANSLATION
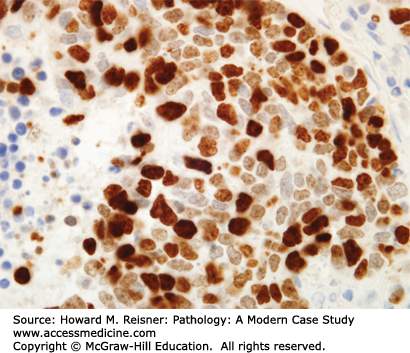
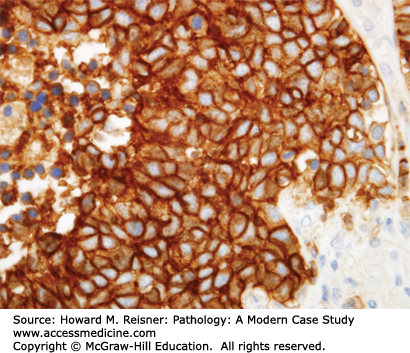
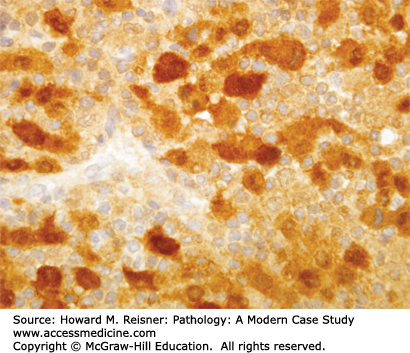
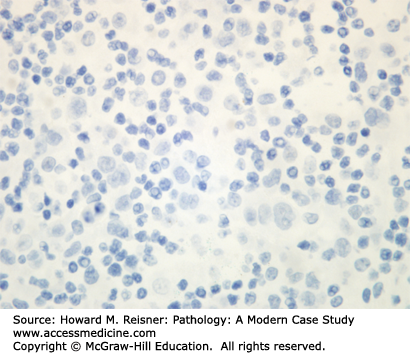
At the histologic level, small cell carcinoma cells are undifferentiated, show coarse “salt and pepper” chromatin, lack nucleoli, and demonstrate numerous mitotic figures and coagulative tumor cell necrosis (Figure 8-10). SCLC shows a high percentage of cells in the growth-division cycle (growth fraction), as estimated by Ki-67 immunohistochemistry (Figure 8-11). At the protein level, most cases express pan-CK, TTF-1, and one or more neuroendocrine markers (CD56, synaptophysin, or chromogranin) (Figures 8-12 and 8-13). Therefore, immunohistochemical panels that include CD56, synaptophysin, or chromogranin will help to distinguish small cell carcinoma from NSCLC (which typically does not express neuroendocrine proteins). The prevalence of synaptophysin and chromogranin expression is lower for SCLC than for well-differentiated neuroendocrine neoplasms such as carcinoid tumors. At the cytogenetic level, there is aneuploidy with marked copy number variation across multiple chromosomes. At the molecular level, P53 mutation and myc amplification are common.
The cell of origin for small cell carcinoma is unclear, but the occurrence of combined SCLC/NSCLC suggests that it may arise from a pluripotent cell type in the bronchi. By definition, small cell carcinoma is an undifferentiated neoplasm that demonstrates neuroendocrine features by H&E and immunohistochemistry.
Like the non-small cell carcinomas, prognosis for small cell carcinoma is related to stage at presentation, and to performance status. Genetic factors are not currently of use as prognostic variables. Because most small cell carcinomas present at a late stage (i.e., with distant metastases), overall prognosis for patients with small cell carcinoma is poor, with roughly 5% 5-year survival rates. In spite of this dismal prognosis, modern chemoradiotherapy has improved initial response rates and significantly prolonged survival, now averaging 18 months.
PLEURAL NEOPLASMS
Malignant mesothelioma was a relatively rare neoplasm prior to the use of mined asbestos starting in the early 20th century. Purified asbestos was heavily used in the manufacturing trades as a flame retardant and thermal insulator, and found high demand during World War II in the shipbuilding industry. Unfortunately, increased numbers of asbestos workers presented with pleural fibrous plaques and malignant neoplasms 20–40 years following exposure to inhaled asbestos. This increased relative risk of pleural plaques and malignant mesothelioma was causally associated with asbestos, and led to the demise of the asbestos industry in the western world. Patients have a poor prognosis without treatment. Combination surgery, chemotherapy, and radiotherapy are currently used to manage these patients.
CASE 8-3
Clinical: A 72-year-old man nonsmoker with progressive dyspnea. PMHx (+) for previous employment as a pipefitter in a shipyard during World War II.
Radiology: PA chest X-ray shows a large pleural effusion. CT scan shows circumferential pleural thickening associated with pleural effusion.
Course: A video-assisted thoracoscopic surgery (VATS) biopsy was performed.
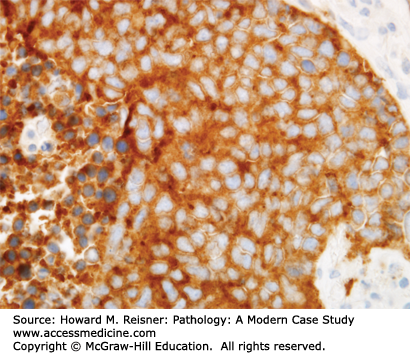
Pathology: H&E-stained section shows a stromal-invasive malignant neoplasm with epithelioid cytology, without specific features of NSCLC (Figure 8-14). These features support a diagnosis of carcinoma or mesothelioma. Immunostains show reactivity for calretinin (Figure 8-15) and CK5/6, with no reactivity for TTF1 (Figure 8-16) or P63. The final diagnosis is epithelioid malignant mesothelioma.
Both the fibrous plaques and the invasive malignant mesothelioma are frequently associated with asbestos fibers (asbestos bodies when seen in tissue) (Figure 8-17) from inhaled dust that migrated out to the subpleural stroma. The mechanism of malignant transformation is unknown, but these fibers presumably predispose the patient not only to a fibrous stromal response but also to the risk of transformation of mesothelial cells into a clonal neoplasm with invasive potential, that is, mesothelioma. At the histologic level, there is overlap in the cytologic features of reactive mesothelial atypia and malignant mesothelioma cells, so the pathologist requires evidence of definite stromal invasion and supportive radiographs to be confident in the diagnosis of invasive malignant mesothelioma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree