Pathologic Examination of Breast and Lymph Node Specimens, Including Sentinel Lymph Nodes
SYED A. HODA
ERIKA RESETKOVA
The purpose of this chapter is to highlight clinically important aspects of the pathologic examination of breast specimens. It is not intended to be a presentation of all differing points of view, nor should this material be regarded as a laboratory “workbook.” The handling and examination of breast specimens should continually evolve to reflect changes in the understanding of breast diseases and alterations in clinical practice. As an example, neoadjuvant chemotherapy is an increasingly exercised option for the management of not only locally advanced but also primary breast carcinoma. Neoadjuvant chemotherapy may “down stage” larger carcinomas. It allows for breast conservation in cases where it might otherwise not be possible, and the assessment of tumor response to neoadjuvant chemotherapy also offers prognostic information. The examination of specimens obtained after neoadjuvant chemotherapy presents a particular set of challenges.
The complexity of pathology reports has increased substantially in the last three decades to accommodate the need for detailed individualized information about breast and related specimens. This has been necessitated by the availability of, and the need for, increasingly personalized medicine, that is, a course of tailored treatment most likely to be beneficial for a given patient with a particular tumor. Consequently, a greater number of findings are now recorded for each specimen. With the routine establishment of sentinel lymph node (SLN) biopsy, greater attention is also given to the handling and evaluation of lymph nodes. Immunohistochemical and other emerging procedures have extended the pathologist’s role to assessing biologic as well as morphologic prognostic markers, and most of these results also appear in the routine pathology report.
Various structured forms of pathology reports have been devised to present the data that a pathologist documents by gross and microscopic examination. These reports have the advantage of ensuring that key observations are reported. They assist the clinical staff by providing comprehensive documentation, and can be the source of a database for studies. A disadvantage of most structured reports is that they are inflexible with the order in which information is presented, in that regardless of its importance for an individual patient a particular finding always appears in the same part of the report. This requires that the entire report be read to ensure that a critical finding that might appear near the end of the report is not overlooked. Some reporting formats have addressed this issue by offering a free-text summary of the diagnosis that highlights the most significant findings as well as a formatted full listing of observations. Additional information about pathology reporting—including information on the reporting of newer clinically relevant data, various staging dilemmas, and significant unexpected findings—may be found in several reviews and position papers.1,2,3,4,5,6,7,8,9,10,11
NEEDLE CORE AND INCISIONAL BIOPSY
Needle core biopsy (NCB) or incisional biopsy specimens should be processed for histopathologic examination in their entirety. Those who perform the biopsy procedure must exercise care not to crush the specimen. An electrocautery-type scalpel should not be used to perform an incisional biopsy (see subsequent text). These samples can be examined by frozen section, but it must be understood that limited information about the lesion is typically obtained from such small specimens. The histologic details can be altered by the frozen section process, and this may impede interpretation of subsequent permanent sections. Consequently, frozen section examination (FSE) is not recommended for incisional biopsy or NCB samples unless there are exceptional clinical circumstances. The samples are suitable for immunohistochemical analysis if not damaged by cautery and if appropriately fixed. Unless frozen section or some other study that requires fresh tissue is intended, small (needle core and incisional) biopsy specimens should be placed immediately in fixative after acquisition.
Newer vacuum-assisted needle biopsy devices (Mammotome, Suros, Breast Lesion Excision System, etc.) are more effective than older NCB instruments for the sampling calcifications,12 and are also being used to “excise” lesions proven
in an earlier biopsy to be benign, for example, fibroadenoma, radial sclerosing lesion, papilloma.13 The advantages of such radiologically conducted excisions include shorter procedure, minimal anesthesia, low complication rate, gratifying patient tolerance, and negligible cutaneous scarring. On the other hand, a major disadvantage is the removal of lesion in a “piecemeal” manner, which precludes assessment of totality of excision and adequacy of margins in the event that carcinoma is detected, and may also preclude the precise assessment of the extent of invasive carcinoma.14
in an earlier biopsy to be benign, for example, fibroadenoma, radial sclerosing lesion, papilloma.13 The advantages of such radiologically conducted excisions include shorter procedure, minimal anesthesia, low complication rate, gratifying patient tolerance, and negligible cutaneous scarring. On the other hand, a major disadvantage is the removal of lesion in a “piecemeal” manner, which precludes assessment of totality of excision and adequacy of margins in the event that carcinoma is detected, and may also preclude the precise assessment of the extent of invasive carcinoma.14
EXCISIONAL BIOPSY
Many factors influence the manner in which excisional biopsy specimens are handled. Paramount among these are logistical considerations relating to when and where the procedure was performed and the particular clinical circumstances. Hence, the material presented in this section should be regarded as guidelines that may need to be modified in some situations. Additional perspectives on handling excisional biopsy specimens can be found elsewhere.15
Gross Examination of Excisional Biopsy
The pathologist is responsible for the detailed description of tissue removed from a patient. This can most accurately be accomplished if the excised specimen is delivered to the pathology laboratory intact, promptly, and unfixed. The pathologist should ensure that all initial handling, including specimen accessioning and preliminary gross examination of breast excisional specimens, be expedited so as to minimize “cold ischemia time.” The latter term refers to the time interval between specimen removal and formalin fixation, which should be kept under 1 hour as recommended by American Society of Clinical Oncology and the College of American Pathologists (ASCO-CAP).16 Minimizing cold ischemia time optimizes not only histologic preparations but also key immunohistochemical and in situ hybridization studies.17,18,19
The size of an excisional biopsy specimen should be recorded in three dimensions, and the general shape (e.g., ovoid, spherical) should be described. Since the overall dimensions of an excisional biopsy specimen cannot be reliably determined after the tissue has been sliced open or dissected by the surgeon, the specimen should be intact when delivered to the laboratory. Contrary to traditional thinking, formalin fixation does not “shrink” excisional biopsies of breast,20 although specimen distortion can occur. In the latter event, specimen dimensions can be misleading vis-àvis orientation. Recording of the “actual specimen volume” using water displacement technique has been proposed as the “gold standard” for certain oncoplastic surgeries wherein volume considerations are important.21 For all other specimens, documentation of dimensions and weight of breast specimens should suffice.
Ideally, the intact excisional biopsy specimen should be promptly delivered to the pathology laboratory. It is preferable that the tissue be unfixed in order to not preclude the possibility of performing a frozen section or to obtain material for other studies. If a delay is anticipated, the tissue may be chilled (in a refrigerator or placed on ice), but freezing of the entire specimen compromises histologic examination. Even if a frozen section is not requested, the tissue should be examined promptly by a pathologist to determine whether a tumor, that is, a grossly identifiable lesion, is present. If a tumor is found, the size should be recorded in three dimensions. Because of the critical prognostic significance of tumor size, this measurement should be made prior to removing for frozen section or other studies. It may be difficult to accurately measure the tumor if the specimen is received previously sliced or otherwise disrupted. The gross character of the tumor (shape, consistency, appearance of cut surface) should be described. Whether or not a distinct lesion is found, the appearance of the native breast parenchyma should be noted (consistency, relative proportions of fat and fibrous tissue, cysts or other lesions).
Intraoperative Evaluation, Including FSE
Presently, the majority of breast carcinomas are diagnosed prior to surgery by an NCB procedure. As a consequence, FSE of a primary tumor is a relatively less commonly requested procedure than in previous decades, and is likely to be done only if samples obtained by the needling procedure are not diagnostic or if the presence of invasive carcinoma could not be determined in the prior sampling. In the absence of a grossly apparent mass, routine FSE of a seemingly benign breast biopsy specimen is not recommended. Although a small proportion of such specimens harbor grossly inapparent in situ or invasive carcinoma, these foci will usually not be detected in the random frozen section.22,23
The appropriateness of FSE for the diagnosis of nonpalpable, mammographically detected lesions has been a subject of controversy. Some reports encompassing large groups of cases have described little difficulty in performing FSE in this setting.24,25,26 Others have recommended that FSE not be routinely performed on such specimens because the lesions are likely to present problems in diagnosis, the tissue may be distorted by freezing and could be rendered more difficult to interpret in permanent sections, and some portion of the tissue may be irretrievably lost in the process of preparing frozen sections.1
Those who recommend using FSE to evaluate nonpalpable lesions caution that a frozen section diagnosis of in situ carcinoma or of a benign lesion should be regarded as “preliminary” because of the potential for sampling. This issue was studied by Niemann et al.,27 who compared the results from 440 consecutive biopsies, of which 98% were examined by frozen section, with those from 604 biopsies, among which only 310 with gross lesions larger than 1.0 cm were submitted for frozen section. In the first group, the false-negative rate was 3.3%, with a sensitivity of 84%, whereas in the second more-selected series the false-negative rate was 1%, with a sensitivity of 96%. The authors concluded that “frozen section examination should be limited to cases with distinct gross lesions >1.0 cm.”
FSE is recommended only if the resultant diagnosis will have an immediate effect on the surgical management, and it is not recommended for the diagnosis of nonpalpable mammographically detected lesions or for biopsy specimens in which a distinct lesion cannot be identified. It is preferable to limit FSE to tumors 1 cm in size or larger, and in all instances a portion of the grossly evident lesion should not be frozen.
The use of touch imprint cytology (TIC) can be a valuable adjunct to FSE, and it has been advocated not only in grossly evident lesions but also for the routine assessment of lumpectomy margins.28 It should be noted, however, that TIC alone can lead to false-negative results in lower-grade carcinomas and invasive carcinoma. The decreased sensitivity of TIC for lobular carcinoma has been reported with regard to the assessment of margins29 as well as SLNs.30
Sampling of the Excisional Biopsy (Lumpectomy) Specimen
The number of samples that should be taken for histologic examination from an excisional biopsy specimen varies greatly with the clinical circumstances, gross appearance of the tissue, and the results of a frozen section, if performed. No fixed rule (e.g., “x” number of samples should be examined per 5 g or cm3 of tissue) can be reasonably applied to all specimens. The tissue used for frozen section must be saved, processed into a paraffin-embedded permanent section, and identified by a term such as the “frozen section control” (FSC). Distinct tumors that appear grossly to be carcinomas 2 cm in size or smaller in greatest extent should be entirely submitted for histology, with samples taken to demonstrate peripheral features of the tumor. In general, tumor tissue should be “banked” only if the distinct lesion exceeds 2 cm in greatest extent. Adjacent breast tissue must also be sampled adequately to enable histologic evaluation for evidence of tumor emboli in lymphovascular channels, in situ carcinoma outside the lesion, and the status of surgical margins.
When no distinct tumor is present, some advocate processing the entire specimen. This may be appropriate in selected situations, owing to clinical, radiologic, or pathologic findings. However, the cost of this approach can become prohibitive, and judgment must be exercised. Specimen radiography can be helpful in this regard, particularly if the targeted lesion had been radiologically detected. This technique has been shown to reliably confirm the excision of the target in 99% of cases31 and contribute to the assessment of resection margins without increased sampling.32 To establish criteria for sampling grossly negative breast biopsies, Schnitt and Wang33 carried out a retrospective study of 384 specimens entirely submitted for histologic examination from biopsies performed for clinically palpable lesions in which no tumor was evident on gross pathologic examination. One to 80 blocks were required to submit entire specimens, resulting in 3,342 blocks (average, 8.7 per case; median, 6 per case). Carcinoma was found in 23 specimens (6%) and atypical hyperplasia in 3 others (0.8%). Eighty percent of the blocks consisting of fibrous parenchyma contained all carcinomas, and two of the three atypical ductal hyperplasias (ADHs). One focus of atypical lobular hyperplasia (ALH) was present among the 20% of the specimens, which consisted entirely of fat. If sampling had been limited to five blocks per case, 41% fewer blocks would have been prepared, but 6 of the 26 (23%) significant lesions would have been missed. By submitting up to 10 blocks of fibrous parenchyma per case, it would have been possible to detect 25 of the 26 significant lesions, with an 18% reduction in blocks. Only a single microscopic focus of lobular carcinoma in situ (LCIS) was overlooked by this selection. Mathematical analysis indicated that “it is not necessary to increase proportionately the actual number of blocks submitted to achieve the same probability of detecting carcinoma or ADH in larger specimens as for smaller specimens.”33 The authors recommended submitting up to 10 samples of fibrous parenchyma or, if the specimen is entirely fat, a similar number of samples. If carcinoma or ADH is found in the first set of slides, the remaining tissue may be processed.
Owings et al.34 investigated the problem of tissue sampling from needle localization excisional breast biopsies. They examined 157 consecutive specimens, among which 32% contained carcinoma. All specimens were entirely submitted for histologic examination. Forty-nine of 50 (98%) carcinomas and 14 of 19 (74%) ADHs were directly related to mammographically detected foci of calcification. All carcinomas and 17 of 19 (89%) ADHs were detected in samples selected from regions of calcification and all fibrous parenchyma.
Although the data from the foregoing studies provide useful guidelines for initial tissue examination, it may be necessary to obtain additional samples to determine the extent of the lesion if initial sections show carcinoma or atypia. The margins of excision should be examined at the time of initial tissue evaluation. These samples may, of necessity, consist of fatty, nonfibrous breast tissue. It should also be remembered that excisional biopsies obtained from elderly women are generally fatty, and those from younger women are mostly fibrous.
Reexcision specimens obtained because a prior biopsy procedure had microscopically positive or close margins may not have grossly apparent tumor. Abraham et al.35 reviewed 97 grossly negative reexcision specimens from which all tissue was submitted for histologic examination to develop guidelines for processing such specimens. Overall, 1,867 tissue blocks were processed (range, 3 to 74; mean, 19.2). Ten or more blocks were prepared in 67% of the cases. Residual in situ or invasive carcinoma was present in 47 reexcisions (48%). The number of blocks with residual carcinoma ranged from 1 to 41, representing 2.4% to 100% of blocks of reexcision tissue. The authors calculated that submitting two sections per centimeter of maximal tissue dimension from grossly benign reexcision specimens detected 97% of lesions having a major clinical impact on treatment with 315 (17%) fewer paraffin blocks.
Issues relating to the processing and reporting of specimens after neoadjuvant therapy have been addressed in published recommendations.36 The fundamental principles that ought to guide the evaluation of such specimens can be summarized as follows: (1) the clinical history of neoadjuvant
chemotherapy, including the specific agents used, must be provided by the surgeon; (2) the site of primary tumor (tumor bed) must be identified grossly; (3) the tumor bed must be adequately sampled; and (4) the pathology report must include assessment of residual invasive and in situ carcinoma, tumor size, cellularity, lymphovascular channel involvement, and margins of excision. The effects of therapy in the breast and lymph nodes should be recorded. The assessment of hormone receptors, proliferation rate via Ki67, and human epidermal growth factor 2 (HER2) status is indicated but may not be possible when scanty tumor remains.
chemotherapy, including the specific agents used, must be provided by the surgeon; (2) the site of primary tumor (tumor bed) must be identified grossly; (3) the tumor bed must be adequately sampled; and (4) the pathology report must include assessment of residual invasive and in situ carcinoma, tumor size, cellularity, lymphovascular channel involvement, and margins of excision. The effects of therapy in the breast and lymph nodes should be recorded. The assessment of hormone receptors, proliferation rate via Ki67, and human epidermal growth factor 2 (HER2) status is indicated but may not be possible when scanty tumor remains.
Gross Description of Excisional Biopsy
The gross description of an excisional biopsy specimen is part of the pathology report. Included in this section must be an index of the tissue samples taken for microscopic examination, indicating the number of tissue blocks and providing a key to explain abbreviations used, if any, to designate individual samples. Each sample taken from a specimen should be identified with a unique letter and number, which ought to appear on the paraffin block and corresponding histologic slide. All samples taken from a specimen should be identified with a common letter, and each should be further labeled with a subnumber. For example, in this system a left breast biopsy specimen would be recorded as specimen “A” and the samples in paraffin blocks and corresponding histologic slides designated as “A1,” “A2,” etc. A second biopsy specimen of the right breast would be listed as “B,” with samples in paraffin blocks and histologic slides designated as “B1,” “B2,” etc. Alternatively, specific designations such as “A-MM,” instead of consecutive letters, which might indicate “Medial Margin,” should be recorded in the aforementioned index. This information is essential to understand the significance of individual slides and the relationship of the findings in these slides to the overall diagnosis.
Some pathology reports do not provide an adequate index of samples (tissue blocks and slides) from a specimen, or do so in a confusing manner. Because of the growing mobility of patients and their slides, it is no longer acceptable to adhere to idiosyncratic labeling practices unique to a given facility. One unsatisfactory method is to label specimen samples taken for histology with consecutive letters without further differentiation. If presented with the slides labeled “A-P,” a pathologist may not have any indication that they represent multiple specimens, conceivably from both breasts. Even with a copy of the pathology report in hand, it requires close attention to segregate the slides corresponding to each specimen. This task would be facilitated by numbering specimens separately as described previously.
Assessing Lumpectomy Margins
The definition of an adequate surgical margin remains elusive, and the extent of acceptable clearance varies considerably. The presence of invasive carcinoma at ink is regarded as a positive margin. Invasive carcinoma at the inked margin is associated with increased ipsilateral tumor recurrence.37 Ductal carcinoma in situ (DCIS), particularly of the micropapillary type, can exhibit “discontinuous spread,” and a more generous extent of margin clearance may be required.38
Technical factors complicate the microscopic evaluation of margins. The surface is often irregular, and it has a large area relative to the sampling that can be represented even in multiple histologic sections. The uneven surface sometimes contains defects or crevices that obviously do not represent the true margin. Definitions of positive and “close” margins have not been standardized. Techniques based on optical scatter spectroscopy that are independent of pathologic examination show potential for intraoperative surgical guidance of margin assessment,39 but it is unlikely that this methodology will be adopted in the near future.
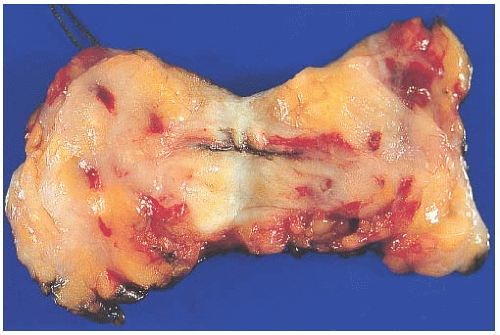
Because the contours and orientation of tissue slices may be altered in the course of preparing histologic sections, it is necessary to mark surfaces corresponding to the margin so that they can be identified microscopically. This is most easily accomplished with finely particulate reagents such as India ink or similar dyes that remain adherent to the tissue throughout processing and are visible through the microscope. Various colors can be used to designate specified margins. If applied carefully, the ink or dye is unlikely to seep into crevices in the tissue surface. The tissue should not be dipped into ink or dye, since this practice leads to seepage into crevices in the surface that can be mistaken for margins microscopically. The pigments adhere better to fresh tissue that has been blotted free of blood than to formalin-fixed tissue. If properly applied to the intact specimen, the surface pigment should not contaminate the interior of the specimen (Fig. 44.1). An excisional biopsy specimen that has been previously sliced by the surgeon cannot be reliably reassembled in order to “ink” the margins. This is an important reason for submitting intact biopsy specimens to the pathologist.
Once “inked” and the blotted dry, the specimen should be serially incised in a plane that will transect the longest palpable dimension. The gross impression of the relationship of an evident tumor to the margins should be reported, although this may underestimate the frequency of involved margins because microscopic foci of invasive and in situ carcinoma at the margins are usually not grossly apparent.40,41 Specimen radiography is not a reliable method for assessing the margins of excision at the time of operation.42 Frozen sections of margins are not indicated unless the tumor appears grossly close to a margin and confirming this intraoperatively will have an immediate impact on treatment. Random frozen sections of margins that appear grossly unremarkable are not recommended. In one study, the sensitivity of this procedure was only 77%.43 Notwithstanding the reportedly successful experience of routine frozen sections for margin evaluationinselectedsettings,44,45 thislaborintensiveandtime-consuming practice is impractical for most laboratories.
Weber et al.46 described the results of a study in which frozen sections were performed on samples obtained from the surface of the cavity left after an excisional biopsy. Five samples were taken from each biopsy cavity in 140 cases. Carcinoma was found in one or more biopsy cavity samples from 21 of the patients (15%). In 14 of the 21 cases, negative
margins were achieved by reexcision. Three patients found to have persistent involvement of margins underwent immediate mastectomy. This procedure was questioned by Esserman and Weidner47 on its cost-effectiveness, difficulty of distinguishing atypical hyperplasia from DCIS, and conversion of planned excisional surgery to an immediate mastectomy.
margins were achieved by reexcision. Three patients found to have persistent involvement of margins underwent immediate mastectomy. This procedure was questioned by Esserman and Weidner47 on its cost-effectiveness, difficulty of distinguishing atypical hyperplasia from DCIS, and conversion of planned excisional surgery to an immediate mastectomy.
It is important to distinguish between pathologic “shaved” and “perpendicular” margins when reporting the margin status of a biopsy. A shaved margin is a thin slice of tissue taken parallel to the inked surface of the specimen as a separate sample. Shave samples are sectioned en face, and the margin is considered positive if carcinoma cells are detected anywhere in the corresponding histologic section. It is important that the inked aspect of such shaved margins be sectioned for microscopic evaluation to be representative of the true margin. Samples designated as “inked” margins
are taken perpendicular to the surface and considered to be positive when carcinoma cells are seen microscopically at the inked edge.
are taken perpendicular to the surface and considered to be positive when carcinoma cells are seen microscopically at the inked edge.
A surgical “shaved” margin (also known as biopsy cavity margins) is a sample obtained by a surgeon intraoperatively from the exposed surface of the biopsy cavity. In recent years, the practice of taking multiple “cavity margins” has gained wide acceptance.48 Cavity margins help in determining the true status of margins by averting the histopathologic pitfalls associated with interpretation of margins in the main lumpectomy specimen—such as ink trickling into crevices and cautery artifact. As many as six cavity margins can be taken, although some surgeons49 may not take an additional anterior margin and posterior margin if these surfaces abut the overlying subcutaneous tissue or underlying fascial plane, respectively. Biopsy cavity margins should be submitted as independent specimens that are distinct from the lumpectomy specimen.
Biopsy cavity margins can be oriented with a suture, clip, or ink to indicate the final margin and processed histologically with this orientation in mind. These additional margin samples have proved to be useful for patients with a positive lumpectomy margin because reoperation is often not necessary if the biopsy cavity margins are negative.50,51,52 Cao et al.52 found that 52 of 103 patients (50.5%) had carcinoma at or within 2 mm of a lumpectomy margin, leaving 51 (49.5%) with negative lumpectomy margins. When biopsy cavity margins were taken into consideration, 61 patients (59%) had negative final margins. As a result nine additional patients did not require reexcision of the biopsy site. Features associated with finding residual carcinoma in biopsy cavity margins were high-grade carcinoma, extensive DCIS in the lumpectomy specimen, and multiple involved lumpectomy margins. Women with positive biopsy cavity margins had a lower mean age at diagnosis (55.2; range, 31 to 88 years) than did those with negative margins (60.6; range, 42 to 85 years).
The presence of negative lumpectomy margins does not provide complete assurance that all carcinoma in the region of the primary site was removed. Evidence for this comes from the analysis of biopsy cavity shave margins obtained from patients with negative lumpectomy margins. Guidi et al.53 found that 39% of patients with a positive shave margin had negative lumpectomy margins, and that the likelihood of having a positive lumpectomy margin increased with more frequent positive shave margins in a given case. Rubin et al.54 reported finding carcinoma in 9% of tumor bed biopsies from 135 consecutive patients with histologically negative margins in the lumpectomy specimens. Cao et al.,52 who considered a lumpectomy margin to be negative if carcinoma was more than 2 mm from the margins, reported that 9.7% of patients with negative lumpectomy margins had carcinoma in biopsy cavity shave margins.
Various technical circumstances may create false-positive lumpectomy margins. Ink that seeps into defects in the tissue may come in proximity with carcinoma that is not at the true margin on the tissue surface.7,52 Detached fragments of carcinoma displaced into ink can be misinterpreted as a positive margin. There is also evidence that compression of excised breast tissue during specimen radiography may create falsepositive lumpectomy margins by compressing normal tissue at the surface of the specimen.52,55 Tissue compression during specimen radiography invariably leads to some degree of “pancaking” of lumpectomy tissue.56 The latter phenomenon generally leads to artifactual reduction of margin clearance of two (compressed) margins and artifactual increment in margin clearance of the other four (expanded) margins. Dooley and Parker55 studied this phenomenon in a series of 220 lumpectomy specimens that were consistently oriented during specimen radiography so that compression was applied only to the skin surface and deep margin. Reexcisions were performed in cases with close or positive margins. No residual carcinoma was found in 12 cases with a close or positive deep margin. When other margins were involved, carcinoma was found in 5 of 14 (35.7%) reexcisions. These results were interpreted as evidence that compression of the deep surface created false-positive margins in this portion of some specimens.
It is not possible for a pathologist to accurately orient the margins of an excisional biopsy specimen without appropriate guidance from the surgeon. This can be easily accomplished if the surgeon places a short suture at the superior margin and a long stitch laterally (Fig. 44.1). The use of orienting sutures has been widely adopted; however, this practice has been shown to have an overall “disorientation” rate of 31%, and a remarkably higher rate (78%) on specimens with a volume of less than 20 cm3.57
One widely practiced approach to evaluating margins histologically employs samples taken perpendicular to each of six inked surfaces (superior, inferior, medial, lateral, superficial, and deep), with additional samples of margins determined by the gross findings. Different colors of ink can be used to distinguish individual margins, but a single color will suffice if margin samples are clearly labeled. Typically, at least two perpendicular sections are taken from each margin surface. The recognition of “true” colors can occasionally be a problem under the microscope, for example, interpretation as green at the interface of blue and yellow or mistaking orange or yellow as red. In such situations, review of the colors as they appear in the corresponding tissue block can be helpful.58 As an aside, inking of NCBs before histologic processing has gained acceptance as a method to reduce specimen “mix-up.”59
Carter60 recommended “peeling” the entire external surface from the specimen, a procedure that is technically challenging. Taking “shaved” samples from the specimen surface has been adopted in some laboratories as an alternative to the peel method. Usually, these shaved samples are sectioned parallel to the surface, and the finding of carcinoma is considered a positive margin, although it may not be in contact with ink. Typically, carcinoma will be within 2 mm of the inked surface when present in a shaved specimen margin.
Cytologic methods for examining the margins of resection have been investigated, but none have proven to be more reliable than histologic sections. In particular, the use of TIC alone can lead to false-negative results in lower grade carcinomas, particularly invasive lobular carcinoma of the classical
type. Cox et al.43 employed touch preparations from the surfaces of lumpectomy specimens to assess the margins. There were three false-positive interpretations. FSE yielded five false-negative and no false-positive results in the same series of specimens. Veronesi et al.61 explored the use of the B72.3 as a means of detecting carcinoma cells at the margins of excisional biopsies. Cytospin preparations were made from specimens obtained by scraping the biopsy surface. One significant limitation of this procedure was the fact that only 57% of the primary carcinomas were B72.3-positive. Immunoreactive cells were detected in 33% of the cytospin specimens containing a B72.3-positive tumor, whereas only 12% had histologically positive margins. The clinical significance of finding B72.3-positive cells cytologically on the surface of a specimen with histologically negative margins remains undetermined. England et al.62 also described a method for evaluating the margins of a lumpectomy specimen by cytologic examination of cells obtained by scraping the surface of the tissue.
type. Cox et al.43 employed touch preparations from the surfaces of lumpectomy specimens to assess the margins. There were three false-positive interpretations. FSE yielded five false-negative and no false-positive results in the same series of specimens. Veronesi et al.61 explored the use of the B72.3 as a means of detecting carcinoma cells at the margins of excisional biopsies. Cytospin preparations were made from specimens obtained by scraping the biopsy surface. One significant limitation of this procedure was the fact that only 57% of the primary carcinomas were B72.3-positive. Immunoreactive cells were detected in 33% of the cytospin specimens containing a B72.3-positive tumor, whereas only 12% had histologically positive margins. The clinical significance of finding B72.3-positive cells cytologically on the surface of a specimen with histologically negative margins remains undetermined. England et al.62 also described a method for evaluating the margins of a lumpectomy specimen by cytologic examination of cells obtained by scraping the surface of the tissue.
The interpretation of margins is directed at the distribution of intraductal or invasive carcinoma in relation to the various margins. The proximity of LCIS of the classical type or proliferative lesions to the margin of resection has not been proven to be a risk factor for local recurrence after breast conservation therapy.63 Nonetheless, the presence of classical LCIS should be noted in the report. It has been recommended that margin status should be reported for the pleomorphic variant of LCIS64 and for its florid variant because there is some evidence that it may be a marker for local recurrence (see Chapter 31). A 2-mm clearance can be regarded as “negative” margin in these, as in most other, settings.
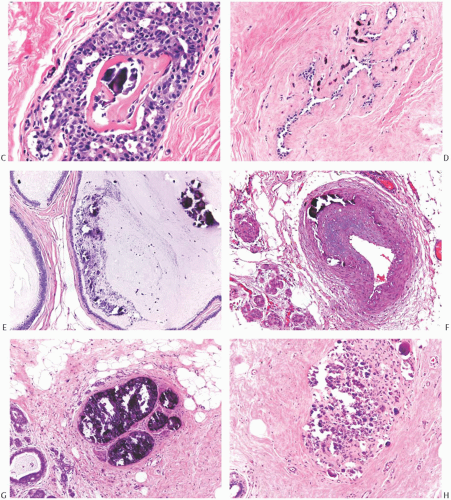
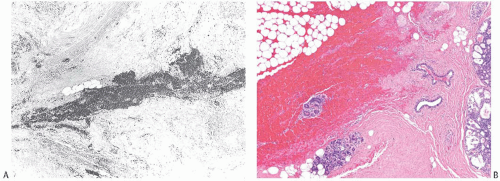
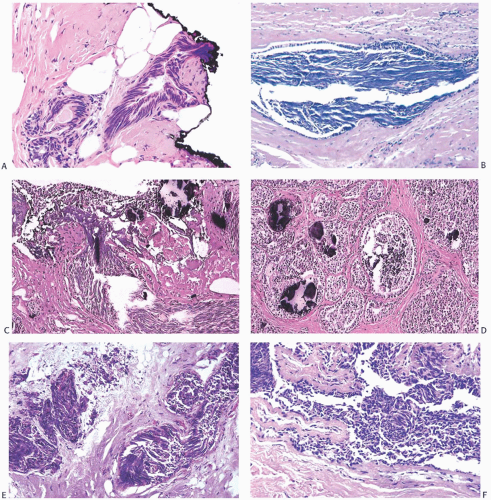
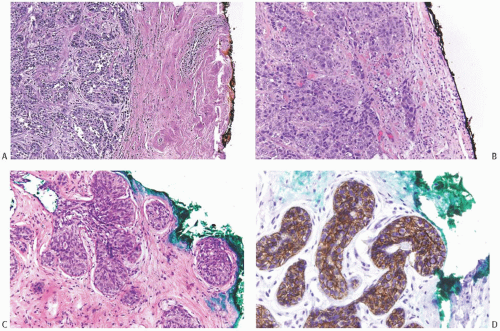 FIG. 44.2. Microscopic appearance of inked margins. A: Invasive ductal carcinoma 1 mm from the inked margin. B: Invasive ductal carcinoma less than 1 mm from the inked margin. C: DCIS of the solid type at the inked margin. The lesion was misinterpreted as LCIS. D: E-cadherin reactivity indicates ductal differentiation in the lesion shown in (C). |
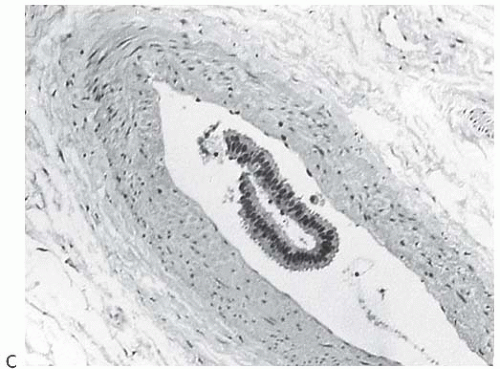
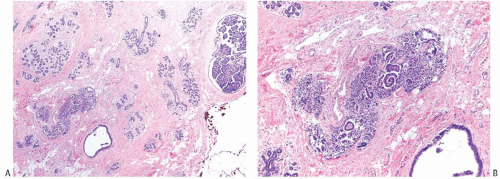
There is no standardized system for reporting the microscopic appearance of margins obtained perpendicular to the tissue surface. Tumor transected at an “inked” surface clearly represents a “positive” margin (Fig. 44.2). This criterion applies equally to in situ and to invasive carcinoma. Borderline situations occur in which tumor closely approaches the margin, but is not transected (Fig. 44.3). One useful convention in these situations is to regard foci less than 1 high-power field as “close to the margin.” Others have defined tumor as close to the margin when it is within 3 mm of the inked surface.65 The actual microscopic distance in millimeters between carcinoma and a margin can be reported. The nature of the carcinoma at or close to the margin (in situ or invasive) should be stated, and some estimate of the extent should be given. It is possible to describe the quantity of carcinoma at or near the margins by specifying the histologic findings in the relevant
slides. For example, the report can be worded as follows: “Invasive ductal carcinoma involves the medial margin along a 1-mm plane and DCIS of the cribriform type is present in rare isolated ducts at the superior and lateral margins. Invasive carcinoma extends to within 1 mm of the lateral margin.”
slides. For example, the report can be worded as follows: “Invasive ductal carcinoma involves the medial margin along a 1-mm plane and DCIS of the cribriform type is present in rare isolated ducts at the superior and lateral margins. Invasive carcinoma extends to within 1 mm of the lateral margin.”
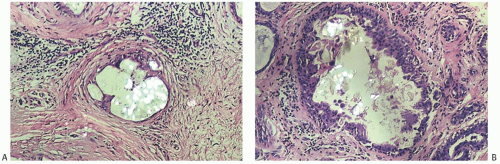
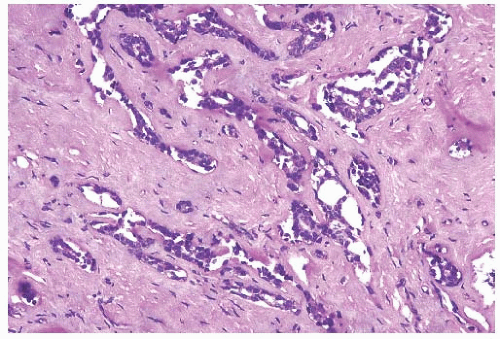
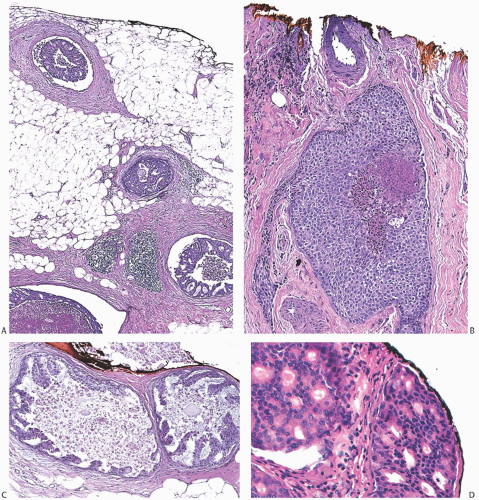 FIG. 44.3. Carcinoma “close” to inked margins. A,B: DCIS less than 1 mm from the inked margin. C: About 0.1 mm of collagen and the basement membrane separate this DCIS from the inked margin. D: Only basement membrane lies between the inked margin and DCIS. Typically, such “close” margins require reexcision to obtain wider “clearance” (usually regarded as 2 mm). |
Clinical Significance of Margin Assessment
The clinical importance of making a distinction between tumor transected at the margin or “close” to the margin is uncertain. Schnitt et al.,66 who defined a close margin as
carcinoma within 1 mm of the inked margin, reported local recurrence rates at 5 years after lumpectomy and radiation for patients with negative and close margins to be 0% and 4%, respectively. In this series, the margin was considered to be “focally” positive when carcinoma was “present at the margins in three or fewer low-power microscopic fields using a 4× objective” and “more than focally” positive if greater than three low-power fields were involved. The local recurrence rates for “focal” and “more than focal” involvement were 6% and 21%, respectively. The presence of an extensive component of DCIS did not significantly increase the risk of local recurrence in patients with negative, close, or focally involved margins. However, the combination of extensive DCIS and more than focal margin involvement was associated with a 50% local failure rate. These observations were confirmed in a subsequent analysis of a larger series by these investigators.67
carcinoma within 1 mm of the inked margin, reported local recurrence rates at 5 years after lumpectomy and radiation for patients with negative and close margins to be 0% and 4%, respectively. In this series, the margin was considered to be “focally” positive when carcinoma was “present at the margins in three or fewer low-power microscopic fields using a 4× objective” and “more than focally” positive if greater than three low-power fields were involved. The local recurrence rates for “focal” and “more than focal” involvement were 6% and 21%, respectively. The presence of an extensive component of DCIS did not significantly increase the risk of local recurrence in patients with negative, close, or focally involved margins. However, the combination of extensive DCIS and more than focal margin involvement was associated with a 50% local failure rate. These observations were confirmed in a subsequent analysis of a larger series by these investigators.67
The number of margins with carcinoma is significantly related to the likelihood of finding residual tumor at the time of reexcision. DiBiase et al.68 reported that the number of positive margins diagnosed in biopsy cavity samples was a significant factor for local tumor control and overall survival (OS). Local control was inferior for women with two or more positive margins when compared to those with negative margins or only one positive margin. An assessment of the margins of excisional biopsy specimens yielded similar data in a study by Papa et al.69 Residual carcinoma was found in 70% of reexcisions after excision with positive margins (tumor at inked surface), and in 25% after excisions with close margins (tumor smaller than 2 mm from inked surface). Residual tumor was found in 12.5%, 37.5%, and 47.9% of reexcision specimens after initial biopsies with 1, 2, or 3 positive margins, respectively. In other reports, residual carcinoma was found in 32% to 62% of reexcision specimens obtained after positive lumpectomy margins.70,71,72,73,74 The likelihood of finding residual carcinoma and the amount of carcinoma in the reexcision specimen were usually a function of the size of the initial tumor and the status of the original resection margins.
A related study of margin status and outcome was reported by Pittinger et al.65 In this analysis, a “close” margin was defined as tumor detected microscopically within 3 mm of the inked margin of the initial excisional biopsy. When a reexcision was performed, residual carcinoma was found in 0%, 24%, 44%, and 48% of patients whose margin status for the initial biopsy was negative, close, positive, and unknown, respectively. A margin of 3 mm or wider has been shown to have a 6.1% (95% CI, 41% to 8.2%) rate of ipsilateral breast events after a median follow-up of 6.2 years in 565 patients with low- to intermediate-grade DCIS not treated with radiation.75 In the same study, the recurrence rate was 15.3% (95% CI, 8.2% to 22.5%) with a median follow-up of 6.7 years for high-grade carcinomas. The authors concluded that 3 mm may be considered as a reassuring extent of clearance in rigorously evaluated and appropriately selected cases but offered the caveat that “further follow-up is necessary to document long-term results.” It is our opinion that it would be premature to draw any conclusions about the long-term outcome of patients in this study because of the relatively indolent clinical course of low-grade DCIS and invasive carcinomas.
The status of lumpectomy margins is an important prognostic indicator for breast recurrence after breast conservation therapy. Multivariate analysis of 869 patients with stage I and stage II breast carcinoma treated by breast conservation with radiotherapy revealed that margin status was the only significant predictor for local control.76 Among women with positive margins, local control was improved when the dose of radiation to the tumor bed was increased (“boosted”). Mansfield et al.77 also found positive margin status to be a significant predictor of local failure in a multivariate analysis of patients with stage I and stage II disease after a median follow-up of 40 months.
The local recurrence rate is lower if margins are negative than if they are positive or unknown,65,78,79 but it is well documented that margins reported to be negative do not provide complete assurance that local recurrence will not occur in patients given equivalent treatment.80 The initial breast recurrence rates for patients treated for “small” invasive carcinomas in a trial comparing lumpectomy to quadrantectomy, in which all patients received radiotherapy, were 8.6% and 4.5%, respectively.61 In a subsequent report, the 10-year estimated breast recurrence rate after lumpectomy was 18.6%, and 7.4% after quadrantectomy.81 Margin status, reported to be positive in 16.3% of patients who underwent lumpectomy and in 4.5% who underwent quadrantectomy, was not significantly related to recurrence in the breast, despite the fact that reexcision was not performed when margins were involved.
A study by the National Surgical Adjuvant Breast and Bowel Project (NSABP)82 revealed a breast recurrence rate of nearly 40% in women with negative margins treated by lumpectomy without radiotherapy. In the NSABP, a margin was negative if tumor was not present at the inked surface. Patients with negative margins who received radiotherapy had a 10% local recurrence rate at 8 years. Others have reported local recurrence rates (after radiation therapy) of 28%,83 13%,84 9%,85 3.7%,79 3%,65 2%,43 and 0%66 when margins were negative. Final margin status that takes into account reexcision or shaved biopsy cavity samples is a more reliable predictor of local control than original excision margins alone.78
Pittinger et al.65 found that after a follow-up of 3 years or more, the frequency of breast recurrence following excision and radiotherapy was the same in those with negative and close margins. The authors concluded that “reexcision of close margins is not necessary in patients” treated by breast conservation therapy. Kunos et al.86 reported that a negative margin with a clearance of 2 mm or more resulted in a significantly lower frequency of breast recurrence (2.1%) than a margin of less than 2 mm in women who also received chemotherapy and hormonal therapy.
Prognostic Significance of Local Breast Recurrence
The relationship of local recurrence in the breast to the occurrence of distant metastases and death due to breast carcinoma is controversial. Several studies have shown no significant difference in distant disease-free survival (DFS) between women who did and did not have a breast
recurrence after conservation therapy. Others have reported a less favorable outcome after local recurrence itself that has been attributed to an initially more aggressive primary tumor causing local as well as distant metastases rather than the local recurrence itself giving rise to systemic disease.87,88,89,90,91,92
recurrence after conservation therapy. Others have reported a less favorable outcome after local recurrence itself that has been attributed to an initially more aggressive primary tumor causing local as well as distant metastases rather than the local recurrence itself giving rise to systemic disease.87,88,89,90,91,92
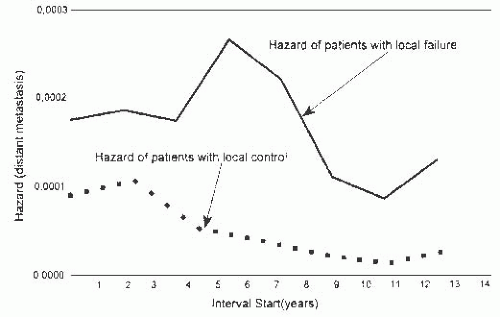
Fortin et al.93 evaluated survival in patients treated by breast conservation with radiotherapy and concluded that local failure could be a source of distant metastases. The study included 2,030 patients with a median follow-up of 6 years. The local control rate at 10 years was 87%. Patients with local failure had a significantly less favorable 10-year survival rate (55%) than those who did not experience a breast recurrence. Local failure was a significant predictor of poor survival in multivariate analysis. The relative risk (RR) of death due to breast carcinoma was 3.6 for women with local recurrence, and their RR of systemic metastases was 5.6, when compared to the RRs for those who did not experience local failure. Additional evidence for local recurrence as a possible source of distant metastases came from analysis of the timing of systemic recurrences. The rate of systemic recurrences was higher among women with local breast recurrences than among those without local failure. The rates of systemic recurrence were parallel for 2 years after treatment, but they rose thereafter among those with local recurrence, reaching a peak around 6 years. By comparison, the group with local control had a declining rate of systemic spread more than 2 years after treatment (Fig. 44.4). Consequently, the mean time until systemic recurrence was significantly shorter among women with local failure (1,050 days) than in the group with local control (1,650 days). In this series, patients with close or positive margins had a higher local failure rate (15.7%) than those with negative margins. The presence of tumor at, or close to, the margin was associated with more frequent systemic recurrence (28%) than negative margins (17%).
Studies of margin status as a predictor of local control vary greatly in terms of the uniformity of surgical procedures performed, the completeness of pathologic evaluation, the forms of adjunctive therapy such as irradiation, and the length of follow-up. Differences can be found in these variables between studies and also in the selection of treatment for patients within a given study. For example, Solin et al.94 treated patients with grossly positive or diffusely positive microscopic margins by mastectomy. Among patients selected for breast conservation, there were significant differences in the total radiation dosages administered to women with negative, positive, close, or unknown margins.
Clinical Follow-up after Breast-Conserving Surgery
Mammography is an important element in the clinical follow-up of patients after breast conservation therapy.95 In one study, 47 of 189 (25%) breast recurrences in patients without systemic metastases were detected by mammography alone. As might be expected, mammographically detected lesions were smaller than those detected by palpation or other signs. Patient outcome was significantly correlated with the size of recurrent tumors. The 5-year frequencies of death and of systemic metastases were 38% and 30.7%, respectively, for patients with tumors 10 mm or less in diameter, whereas for patients with recurrent tumors larger than 10 mm, the frequencies were 46% and 54.4%, respectively. These results suggest that the detection of breast recurrence as soon as possible after conservation therapy might be beneficial to the overall prognosis in this group.
Reexcision of Biopsy Site
Reexcision of the biopsy site is indicated when the margins of the initial excision are involved grossly by carcinoma, if breast conservation is desired, and if an acceptable cosmetic result can be achieved. Other relative indications for reexcision are the finding of residual microcalcifications at the biopsy site in the postbiopsy mammogram, carcinoma microscopically at or close to the margins, the presence of “extensive” intraductal component associated with in an invasive carcinoma (defined later in text) in the initial excision specimen, and inability to assess the margin status of the first excision. The likely cosmetic effect of reexcision and whether radiation will be employed can further influence the decision to recommend reexcision.
The reexcision specimen should be handled by the surgeon and pathologist in the same fashion as a primary lumpectomy. The specimen should be submitted intact with orientation markers such as sutures, and the external surfaces should be inked in the same manner as in a primary lumpectomy. FSE of the margins of reexcision specimens is rarely indicated except to confirm a gross impression of carcinoma extending to a margin. The gross assessment of the amount of carcinoma, if any, remaining at the biopsy site can
be unreliable because regional fat necrosis, fibrous scarring, and organizing hemorrhage in this region can produce a palpable alteration that may be mistaken for carcinoma. Most reexcision specimens, especially those 3 cm or less in greatest extent, can be entirely submitted for histologic examination. The extent of sampling of larger reexcision specimens depends on the gross findings and the particular indications for reexcision in each case, but should generally follow the guidelines for primary lumpectomy as enunciated above.
be unreliable because regional fat necrosis, fibrous scarring, and organizing hemorrhage in this region can produce a palpable alteration that may be mistaken for carcinoma. Most reexcision specimens, especially those 3 cm or less in greatest extent, can be entirely submitted for histologic examination. The extent of sampling of larger reexcision specimens depends on the gross findings and the particular indications for reexcision in each case, but should generally follow the guidelines for primary lumpectomy as enunciated above.
Extensive DCIS Component of an Invasive Carcinoma
Extensive intraductal component (EIC) of an invasive carcinoma has been defined as the presence of DCIS making up more than 25% of the entire area of the invasive carcinoma and the presence of DCIS in the surrounding breast tissue.2 Residual carcinoma, especially DCIS, is more likely to be found in a reexcision if the primary invasive carcinoma was accompanied by EIC.72 Among patients with a microscopically positive margin in the primary excision, there is a substantially greater likelihood of finding residual carcinoma in the reexcision if the initial specimen has EIC.2 The presence of EIC has been shown to be a predictor of increased risk of local recurrence in the breast by some investigators.2,65,83,96,97,98 Sinn et al.96 defined EIC as being present if the extent of the intraductal component was at least twice the size of the invasive carcinoma or the tumor was predominantly intraductal. The presence of EIC in this study was associated with low tumor grade, positive resection margins, and multifocal invasive carcinoma. In multivariate analysis, the factors associated with local recurrence were EIC (RR, 1.9), high-grade tumor (RR, 1.76), invasive lobular carcinoma (RR, 1.65), age at diagnosis less than or equal to 40 years (RR, 1.39), and “angioinvasion” (RR, 1.34). Others have not reported a higher local recurrence rate in women with EIC, or they have found that it was a significant predictor in univariate but not in multivariate analysis.66,67,78,84
Ohtake et al.99 developed a computerized method for mapping the distribution of DCIS in patients with invasive carcinoma. The procedure used graphics to create a threedimensional reconstruction of the ductal system from information obtained with subgross serial sections of the specimen. In most cases, intraductal extension tended to be distributed from the invasive lesion toward the nipple. Anastomosing ductal branches connecting otherwise independent ductal systems were found in breast tissue not involved by DCIS, and in one case such a connecting branch provided the bridge for extension of DCIS beyond a single ductal system. Further development of such systems could provide a method for mapping DCIS in clinical practice.
Many breast tumors are presently diagnosed initially by NCB, especially when the lesion is not palpable. The samples obtained by this procedure typically include portions of the main lesion as well as peripheral tissue. Jimenez et al.100 reported that the relative proportions of DCIS and invasive carcinoma in needle biopsy samples were significantly correlated with the distribution of these components in corresponding surgical excisions. An NCB was deemed to have EIC if the ratio of ducts with DCIS to the number of cores was greater than 0.5. A specimen with a ratio of 0.5 or less was considered to be EIC-negative. EIC was present in 70% of excisions after core biopsies, with a ratio greater than 0.5, and in 36% when the ratio was less than or equal to 0.5. EIC was present in only 2 of 29 (7%) excisional biopsies obtained after the core biopsy specimen with invasive carcinoma had no DCIS. These findings suggest that describing the proportion of NCB samples involved by DCIS may predict the presence of EIC and be useful for planning the extent of surgical excision.
TNM Staging
Tumor (size), regional node (involvement), (distant) metastases (TNM) staging according to erstwhile criteria of the American Joint Committee on Cancer-Union for International Cancer Control (AJCC-UICC) may be inaccurate when based only on the initial excision if the patient has positive margins and residual tumor is detected in a reexcision specimen. Evidence to support this supposition was presented by Brenin and Morrow,101 who found a significantly greater frequency of nodal metastases in patients who had residual invasive tumor in a reexcision than when no tumor remained. The analysis was controlled for major predictors of lymph node metastases and compared patients on the basis of tumor size measured only in the initial excision. The authors concluded that understaging may occur, especially among patients with T1a-b (i.e., invasive carcinoma measuring 1.0 cm or smaller) tumors, if invasive carcinoma remains in a reexcision, and they suggested this be considered in planning treatment. No method was offered for arriving at a tumor size based on measurements from both the excision and reexcision specimens.
This issue is difficult to resolve, since breast carcinomas are usually not spherical and it is uncertain whether the diameter of residual tumor should be added to the largest diameter of the primary lesion. Any size increment, howsoever minimal, could have a major impact on treatment for example by changing staging from T1a to T1b, or from T1b to T1c. In the latest TNM Staging System, the grossly positive margin of a carcinoma cannot be “T” staged. On the other hand, “T” staging can be based on a measurement of the gross carcinoma when the margins are positive only miscroscopically.101
Refinements in radiographic techniques offer promise for preoperative staging, and have the potential to improve the accuracy of surgical excision. Magnetic resonance imaging (MRI) has proven more useful than mammography for evaluating possible invasion of the pectoral muscle in patients with a deep or posterior tumor. A study of 19 patients with this clinical presentation revealed that 12 had mammographic findings suggestive of muscle involvement.102 MRI showed extension to the prepectoral fat plane and muscle enhancement in five of these cases that proved to be the only ones with muscle invasion demonstrated surgically.
MRI also has the potential for detecting the presence and distribution of EIC or multifocal and multicentric carcinoma. Esserman et al.103 analyzed 44 cases in which MRI and
mammography had been performed. The MRI interpretation was in concordance with the pathologic findings in each of 19 patients with a unicentric tumor, all 10 patients with multifocal or multicentric carcinoma, and in 7 of 8 patients (88%) with EIC. The false-positive rate, representing an MRI determination of more extensive tumor than was detected pathologically, and the false-negative rate were each about 3%. Ultrasonography has also proven to be effective for detecting multifocal or multicentric carcinoma that was not apparent in conventional mammograms.104
mammography had been performed. The MRI interpretation was in concordance with the pathologic findings in each of 19 patients with a unicentric tumor, all 10 patients with multifocal or multicentric carcinoma, and in 7 of 8 patients (88%) with EIC. The false-positive rate, representing an MRI determination of more extensive tumor than was detected pathologically, and the false-negative rate were each about 3%. Ultrasonography has also proven to be effective for detecting multifocal or multicentric carcinoma that was not apparent in conventional mammograms.104
Lymphovascular Tumor Emboli and Breast Recurrence after Conservation Therapy
The presence of lymphovascular tumor emboli associated with a primary carcinoma or in a reexcision specimen was associated with a significantly increased risk of local recurrence in patients treated by lumpectomy and radiotherapy60,61 in some reports,69,82,83,85 but others reported no association.67,95 The influence of radiotherapy and systemic adjuvant therapy on the risk of breast recurrence associated with lymphovascular tumor emboli has not been determined. However, various forms of treatment may be a factor in the different results reported in previously cited studies.
Specimen Radiography
The radiologic examination of excised breast tissue has been employed for nearly 75 years.105 As early as 1913, Salomon,106 a surgeon at the University of Berlin, reported on the use of x-rays to study mastectomy specimens. He employed serial sections of specimens in order to correlate histologic and radiologic features of breast carcinoma. He described radiologically detected calcifications in breast carcinomas, but the clinical significance of this finding was not appreciated until several decades later.107 In 1951, Leborgne108 commented on a case in which “roentgenographic study of the operative specimen also permitted the localization of the tiny calcifications for histopathological study, and thus aided in finding a small cancer.”
The increasing use of mammography is a major factor in the progressively earlier stage of breast carcinomas detected in the last three decades.109 Carcinomas are detected mammographically because of an abnormal soft tissue structure, the presence of calcifications, or both. Soft-tissue alterations in the breast may be distorted in the excised specimen, rendering them less suitable to specimen x-ray study. Changes in the relationships of structures in the compressed breast at the time of mammography and the altered orientation in an excised specimen make it difficult to accurately compare these features in clinical and specimen x-rays. Clinical mammographic findings that do not lend themselves to specimen radiography are alterations in parenchymal pattern, skin changes, vascular abnormalities, and ill-defined lesions.110,111 Specimen compression devices are useful for localizing noncalcified lesions in specimen radiographs,112 and image quality may be improved further if the specimen is immersed in water112,113,114 (Fig. 44.5). Sonography has also been employed to assess biopsy specimens obtained after preoperative sonographic localization of nonpalpable lesions.115
The availability of corresponding mammogram and specimen radiograph is helpful in ensuring radiologic-pathologic correlation (Figs. 44.6, 44.7 to 44.8). The presence of calcifications associated with a lesion provides an intrinsic marker that can be visualized in clinical and specimen radiographs. Specimen radiography provides a method for proving that a nonpalpable lesion has been removed, and it is an efficient technique for pinpointing the area for histologic examination.116
Carcinoma is found in approximately 25% of nonpalpable lesions biopsied because the mammogram reveals a pattern of calcification that suggests carcinoma.110,111,117
Carcinoma is found in approximately 25% of nonpalpable lesions biopsied because the mammogram reveals a pattern of calcification that suggests carcinoma.110,111,117
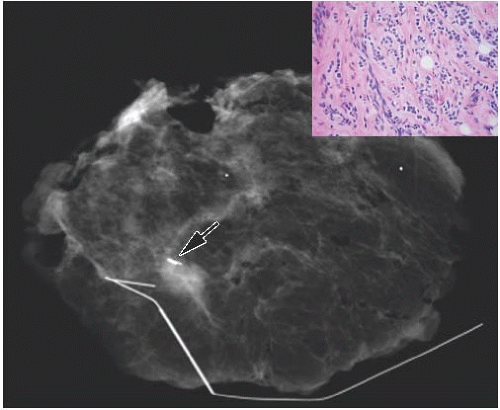
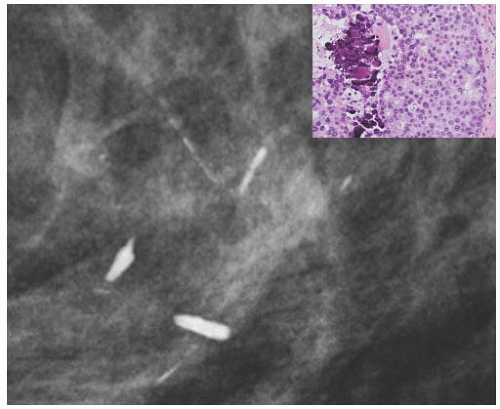 FIG. 44.6. Radiologic-pathologic correlation. Magnified view of a mammogram of the left breast from a 54-yearold woman reveals linear-branching cast-like calcifications that upon excision corresponded to calcifications in DCIS of the solid type with high-grade nuclei and central necrosis (inset). (Courtesy of Dr. Shabnam Momtahen.) |
In order to effectively evaluate a breast specimen radiograph, it is helpful to have the clinical mammogram simultaneously available. A variety of procedures have been described for specimen radiography, and this chapter will not address their advantages or disadvantages, which primarily depend on the availability of personnel, equipment, and other resources in a given institution. Thus, the processing and interpretation of specimen radiographs may be the responsibility of a pathologist, a surgeon, or a radiologist. However, certain principles enunciated below apply in most situations.
An x-ray of the intact excisional biopsy specimen should be obtained, and if possible, it should be compared with the mammogram. If the tissue is dissected prior to obtaining a specimen x-ray, disruption of the pattern of calcifications may occur. Changes in the position, that is, orientation, of the specimen may make it difficult to locate calcifications. Radiographs of NCB specimens should also be obtained (Fig. 44.9).
It is recommended that nonpalpable mammographically detected lesions be processed solely for permanent sections, and that frozen sections be performed only in exceptional situations.1 In one study of 359 mammographically detected lesions, frozen section yielded a correct diagnosis in 68% of cases, 17.3% did not have a frozen section, 1.9% of frozen sections yielded false-negative results, and 0.6% were falsepositive diagnoses.118
The immediate goal of specimen radiography is to confirm that a nonpalpable lesion has been excised. Ideally, this should be determined intraoperatively. If the lesion is not present in the specimen, the surgeon may elect to obtain more tissue at that time or a postoperative mammogram at a later date. Postoperative clinical mammography is reportedly useful to evaluate patients with a negative or inconclusive specimen radiograph following biopsy of a lesion with or without calcifications.119 Others recommend delaying postoperative mammography for 6 to 12 weeks after biopsy to minimize
discomfort associated with the procedure and to allow subsidence of postsurgical inflammation that may obscure the mammogram.120 Missed lesions have been reported in up to 13.6% of needle localization biopsies,121 but in most series this occurred in 5% or less of cases.122,123,124
discomfort associated with the procedure and to allow subsidence of postsurgical inflammation that may obscure the mammogram.120 Missed lesions have been reported in up to 13.6% of needle localization biopsies,121 but in most series this occurred in 5% or less of cases.122,123,124
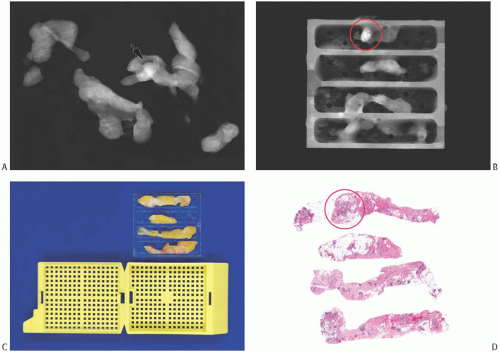 FIG. 44.9. Radiologic-pathologic correlation in a needle core biopsy specimen. A commercially available tissue “tray” can facilitate radiology-pathology correlation mainly by allowing the usually fragile biopsied tissue to maintain its orientation and integrity.160 A: A set of NCB samples in a specimen radiograph. The arrow indicates the suspicious lesion. B: The individual NCB specimens have been placed into one of the four separate slots in the “tray.” This radiograph of the tray indicates the location of the lesional tissue (circle). C: The tray fits into a standard tissue cassette for histologic processing. The biopsies are embedded into the tissue block with the same orientation as in the “tray.” D: The corresponding histologic slides have the tissue samples with similar orientation, allowing ready radiologic-pathologic comparison of the circled calcifications and density. (Courtesy of Dr. O. Tawfik.) |
False-negative specimen radiographs may occur because the lesion has been distorted by the operation, by dissection after excision, or as a result of positioning of the specimen that obscured the lesion when the image was obtained. The specimen radiograph may also be negative because of inaccurate preoperative localization or loss of localization resulting from displacement of a needle or wire used in this procedure. Finally, the position of calcifications may be misjudged in conventional mammographic views if localization has not been performed. Cutaneous calcifications can be misinterpreted as an intraparenchymal lesion.125 Tangential views and stereotactic imaging are useful for confirming the cutaneous position of such calcifications.126 One unusual cutaneous abnormality that may mimic calcifications in the breast is a skin tattoo.127 Tattoo powder applied to specimens to mark margins is an iatrogenic source of microcalcification-like particles that may interfere with the interpretation of a specimen radiograph.128
A variety of procedures are available to mark areas in the breast preoperatively in order to guide the surgeon in excision of the lesion and to minimize the size of the specimen required. These include the placement of one or more needles or hooked wires in proximity to the lesion129,130 and, some years earlier, the injection of dyes.131,132 Presently, almost all localization procedures employ either a wire or a needle placement technique with ultrasound or stereotactic guidance or radioactive seed localization. The latter procedure
is an effective approach, and it may be used in the surgical excision of previously biopsied radiographically identified lesions, and serve an alternative to the placement of marker “clips,” which can “migrate.” Radioactive seed localization is an increasingly utilized approach that is as acceptable to patients, radiologists, and surgeons as the wire or needle localization approach, and it may offer the considerable added benefit of a lower positive margin rate.133 This method of localization has been successfully used to guide surgery following neoadjuvant chemotherapy—in unifocal as well as multifocal tumors.134 The principles for the safe handling of radioactive seeds in breast specimens have been outlined.135
is an effective approach, and it may be used in the surgical excision of previously biopsied radiographically identified lesions, and serve an alternative to the placement of marker “clips,” which can “migrate.” Radioactive seed localization is an increasingly utilized approach that is as acceptable to patients, radiologists, and surgeons as the wire or needle localization approach, and it may offer the considerable added benefit of a lower positive margin rate.133 This method of localization has been successfully used to guide surgery following neoadjuvant chemotherapy—in unifocal as well as multifocal tumors.134 The principles for the safe handling of radioactive seeds in breast specimens have been outlined.135
The various aforementioned techniques for localization assist the surgeon and the pathologist in finding the targeted lesion. Before the position of the specimen is changed after imaging, the site of the radiographically detected calcifications or a density should be identified grossly. This targeted portion of the tissue should be excised en bloc from the specimen and labeled. The remainder of the tissue must also be dissected, since occasionally calcifications lie in a benign process near an unanticipated carcinoma fortuitously included in an excisional biopsy.
It is essential that the localization wire or wires remain within the excisional biopsy specimen when it is delivered to the pathology laboratory. The pathologist must describe the wire or wires in the gross specimen report. It is advisable that the length of wire or wires be measured. Localization wires have occasionally been inadvertently transected intraoperatively, or they may retract into the breast preoperatively and migrate from the lesion in question.136,137 In one remarkable case, a retracted wire reportedly migrated to the subcutaneous tissue of the ipsilateral buttock.137
Most of the lesions that are the target of a wire localization biopsy procedure are microscopic. Unless there is a compelling clinical need, FSE is not recommended in these cases.1 If a decision is made to attempt a frozen section and the diagnosis is not readily apparent immediately, further sectioning should not be carried out and the remaining tissue must be fixed for permanent sections. The surgeon must defer to the pathologist’s judgment as to the feasibility of obtaining a diagnosis by frozen section in this, as in any other, situation.
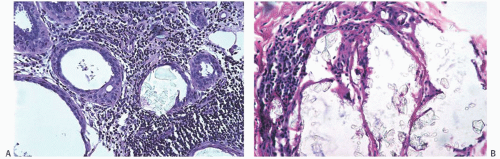
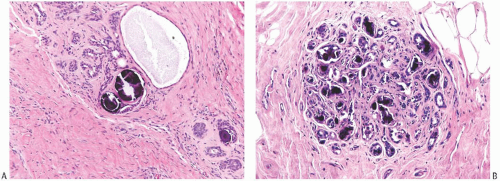 FIG. 44.10. Calcification in breast tissue. Various configurations of phosphate calcifications are depicted. A: Microcalcifications of the psammomatous type in lobules. B: Calcifications in sclerosing adenosis (SA). C: An “ossifying” type of calcification in DCIS. D: Calcifications in extreme glandular atrophy. The patient was a nanogenerian. E: Calcifications of the fine powdery and coarsely granular types are seen in a mucin-filled cyst. A mucocele-like lesion (not shown) was present in the vicinity. F: Arterial intramural calcification. G: Dense stromal calcifications. H: Coarse calcifications in a lobule in a case with complete pathologic response following neoadjuvant chemotherapy. |
The identification of calcifications in NCB specimens can be facilitated by the provision of the corresponding specimen radiograph (Fig. 44.9). The latter also provides evidence for the removal of the target calcification, and guides the pathologist in correlating the microscopic finding with the radiologic appearance of the lesion.
PATHOLOGY OF MAMMARY CALCIFICATIONS
Calcium Phosphate Calcifications
Microcalcifications found in breast tissue were thoroughly described and classified by Frappart et al.,138,139 and the basic characteristics of calcifications in breast lesions were outlined by Tse et al.140 The majority of calcifications detected in mammograms are basophilic concretions of varying size composed of calcium phosphates largely in the form of hydroxyapatite141 (Fig. 44.10). These type II calcifications of Frapport are not birefringent. They react with the von Kossa stain and with alizarin red at pH 4.2 and 7.0.142 A black precipitate is formed with silver nitrate/rubeanic acid, but not after pretreatment with 5% acetic acid.142,143
Foschini et al.144 described the patterns of calcium phosphate calcifications in DCIS. Granular calcifications were formed by deposition of calcium on nuclear debris or on secreted
mucosubstances. Lamellar calcifications resulted from deposits of calcium on proteinaceous or mucoid material arranged in concentric layers. Calcification on nuclear debris was found only in DCIS with necrosis of intermediate or poorly differentiated grade, whereas the other types of calcification were present mainly in well to moderately differentiated lesions.
mucosubstances. Lamellar calcifications resulted from deposits of calcium on proteinaceous or mucoid material arranged in concentric layers. Calcification on nuclear debris was found only in DCIS with necrosis of intermediate or poorly differentiated grade, whereas the other types of calcification were present mainly in well to moderately differentiated lesions.
Calcium Oxalate Calcifications
Type I microcalcifications of Frappart composed of calcium oxalate dihydrate crystals (Weddelite) are birefringent, nonbasophilic, von Kossa-negative crystals141,143,145 (Fig. 44.11). The term “Weddelite” derives from the fact that calcium
oxalate used commercially was originally extracted from the Weddell Sea, located south of the Falkland Islands, named after the British explorer James Weddell (1787 to 1834).146 Calcium oxalate calcifications are not stained by alizarin red at pH 4.2, and at pH 7.0 alizarin red staining is weak or absent. Black staining is observed with silver nitrate/ rubeanic acid, with or without pretreatment with 5% acetic acid.143 Because they are colorless, calcium oxalate crystals are difficult to identify in hematoxylin and eosin (H&E)-stained sections with standard light microscopy. They tend to fragment and are sometimes accompanied by multinucleated giant cells. Intact crystals assume various configurations including overlapping plates, rosettes, sheaves, rods, and geometric shapes such as pyramids or diamonds (Fig. 44.12).
oxalate used commercially was originally extracted from the Weddell Sea, located south of the Falkland Islands, named after the British explorer James Weddell (1787 to 1834).146 Calcium oxalate calcifications are not stained by alizarin red at pH 4.2, and at pH 7.0 alizarin red staining is weak or absent. Black staining is observed with silver nitrate/ rubeanic acid, with or without pretreatment with 5% acetic acid.143 Because they are colorless, calcium oxalate crystals are difficult to identify in hematoxylin and eosin (H&E)-stained sections with standard light microscopy. They tend to fragment and are sometimes accompanied by multinucleated giant cells. Intact crystals assume various configurations including overlapping plates, rosettes, sheaves, rods, and geometric shapes such as pyramids or diamonds (Fig. 44.12).
In one series, 9 of 66 (13.6%) mammographically detected calcifications identified histologically consisted of calcium oxalate crystals, 72.7% were calcium phosphate, and 13.6% were a mixture of calcium oxalate and calcium phosphate.141 Similar frequencies of calcium phosphate and oxalate calcifications have been found by other investigators.147 Tornos et al.143 reported finding calcium oxalate calcifications alone in 2% and in combination with calcium phosphate calcifications in 10.4% of 153 specimens. Calcium oxalate crystals have been responsible for 7.3%145 and 12%148 of mammographically localized calcifications that led to biopsy. Calcium oxalate crystals can rarely appear in fine-needle aspiration (FNA) biopsy specimens of breast, wherein they usually appear in clusters and are strongly birefringent under polarizing microscopy.149
Calcium phosphate calcifications and calcium oxalate crystals appear as conventional calcifications in specimen radiographs and in clinical mammograms. Calcium phosphate calcifications typically have high to medium density, and they may have irregular or distinct shapes suggestive of carcinoma in a mammogram, whereas calcium oxalate crystals are likely to appear as polyhedral deposits of lowto medium density.150 An analysis of 2,000 screening mammograms revealed that 3% of women examined had two or more polyhedral microcalcifications.151
Calcium oxalate crystals are most frequently seen in benign cysts, especially those lined by apocrine epithelium, and in dilated ducts (Figs. 44.11 and 44.12).141,143,145,147,152,153 This association suggests that apocrine epithelium is able to synthesize or concentrate and secrete oxalic acid or calcium oxalate. In some cases, calcium phosphate calcifications have been present coincidentally with calcium oxalate deposits in proliferative lesions. It is unusual for calcium oxalate crystals to develop in carcinoma.139,152 Calcium oxalate crystals have been described in papillary DCIS.154
Calcium oxalate crystals probably account for the majority of instances in which “calcifications” are reportedly not present in histologic sections of breast biopsies obtained for calcifications found in a mammogram.155,156 In this situation, sections should be examined with polarized light. Fragments of birefringent material may be the only residual evidence of larger crystalline deposits that are sometimes shattered or partially dissolved during processing of the tissue.
Localizing Calcifications in Tissue Blocks
Radiographic examination of paraffin-embedded tissue blocks is an essential procedure if a biopsy has been performed for calcifications and they are not evident in the histologic sections.157 Calcifications are easily identified in the resultant x-ray images of the blocks. This allows the pathologist to select blocks for deeper sections. Radiographs should be obtained to document the presence of calcifications in NCB samples.158,159 A novel, recently described device ensures maintenance of orientation of NCB samples from procurement through microscopic evaluation (Fig. 44.9). After mammographic identification of calcifications, the NCBs are harvested, placed in tissue trays, and then x-rayed to confirm calcifications. The tray with the corresponding radiograph is submitted to the laboratory, where the core biopsies are placed into cassettes without “disturbing” the orientation of the cores. This procedure facilitates the ready identification of calcifications in NCB specimens.160
Calcifications may be lost in the course of trimming paraffin blocks that is part of the process of preparing routine histologic sections. Winston et al.161 documented the presence of calcifications in microtome shavings, including some particles larger than the thickness of paraffin sections that were apparently fractured and then knocked out of the tissue by the microtome blade.
The number of sections taken from tissue blocks for the evaluation of calcifications in NCBs remains a matter of some debate. The need to conserve tissue for any immediate or future ancillary studies is a major consideration, as are factors relating to resources and finances. Be that as it may, it is recommended that at least two H&E-stained levels, cut approximately 50 µm apart, be evaluated initially. Additional levels could be obtained if needed. Lee et al.162 have proposed that while the histologic examination of multiple levels is useful in the evaluation of calcifications, the examination of one level alone may suffice for cases in which the biopsies were taken for reasons other than calcifications.
BI-RADS Classification of Calcifications
Image analysis has been used to study calcifications as they appear in mammograms in order to refine the characterization of calcification associated with benign and malignant lesions.163,164,165,166 Features examined included the number of particles in a cluster, the number of clusters, distances between calcifications in a cluster, and the area of clusters. Data from these analyses may lead to the development of automated image screening systems for calcifications, but such procedures cannot replace the interpretation of noncalcific parenchymal alterations.
Calcifications in clinical mammography are currently reported according to the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) classification.167 The BI-RADS lexicon definitions range from category 1 (normal) to categories 5 (highly suspicious) and 6 (histologically proven malignancy). Variability in the application of BI-RADS can lead to classification errors, especially in category 3 lesions, that is, those interpreted to be probably benign, needing short-term follow-up.168
Calcifications classified as ductal are typically linear if associated with carcinoma, and often branching, whereas benign ductal calcifications are described as “rod-like.” Nonductal calcifications are typically punctate, pleomorphic, or coarse irregular deposits. The distribution is usually reported as clustered, linear, segmental, or regional.
Relationships of Calcifications to Primary and Recurrent Carcinoma
The precise microanatomic distribution of calcifications is an important consideration when correlating imaging findings with the pathologic diagnosis. Although the presence of calcifications may contribute to the radiologic impression that leads to a biopsy procedure and the finding of carcinoma, some of the calcifications may not be located in the carcinoma. This becomes a concern especially when an NCB procedure is performed to sample a lesion with intermediate (BI-RADS 3) or suspicious (BI-RADS 4) calcifications. In some instances the needle biopsy sample shown to contain calcifications by specimen x-ray and histologic examination can be an unrepresentative specimen of peritumoral tissue. To assess this issue, Selim and Tahan169 studied the distribution of calcifications in benign tissue surrounding carcinomas and in the carcinomas. Calcification was limited to the carcinoma in 31% of cases and present only in benign components within 1 cm of the carcinoma in 34%. Calcifications were present in the carcinoma and adjacent benign tissue in 35%. These observations underscore the need for correlation between mammography and the diagnosis obtained by NCB to determine whether the sample is representative of the radiographic image. If the benign biopsy diagnosis is discordant with the mammographic impression, surgical biopsy should be considered, even if calcifications were obtained and documented in the NCB.
Calcifications are often an important clue to the presence of recurrent carcinoma in the breast after conservation
therapy. Dershaw et al.170 found that at least 10 calcifications were present in 17 of 22 (77%) breast recurrences. The patterns of calcification were classified as highly suspicious for carcinoma (BI-RADS 5) in 77% of cases and as suggestive of carcinoma requiring biopsy (BI-RADS 4) in the remaining cases. Less worrisome punctate and coarse calcifications were also present in 36% and 14% of cases, respectively, but these were always coincidental with malignant patterns of calcification. Other investigators have not observed the same degree of calcification specificity in the breast after conservation therapy.171 When an NCB sample from the site of a possible breast recurrence shows calcifications and benign histologic changes, the results must be integrated with the radiologic and mammographic findings in order to determine if surgical excision should be performed.
therapy. Dershaw et al.170 found that at least 10 calcifications were present in 17 of 22 (77%) breast recurrences. The patterns of calcification were classified as highly suspicious for carcinoma (BI-RADS 5) in 77% of cases and as suggestive of carcinoma requiring biopsy (BI-RADS 4) in the remaining cases. Less worrisome punctate and coarse calcifications were also present in 36% and 14% of cases, respectively, but these were always coincidental with malignant patterns of calcification. Other investigators have not observed the same degree of calcification specificity in the breast after conservation therapy.171 When an NCB sample from the site of a possible breast recurrence shows calcifications and benign histologic changes, the results must be integrated with the radiologic and mammographic findings in order to determine if surgical excision should be performed.
In recent years, the technique of procuring NCBs of the breast has evolved toward the use of vacuum-assisted devices with larger (8- to 11-G) needles rather than that of semiautomatic guns with smaller (14- to 18-G) needles. Also, markers made of titanium, stainless steel, or carbon-coated ceramic are increasingly being deployed during the procedure to indicate the biopsy site. These markers serve to guide subsequent radiologic studies (mainly for follow-up purposes), surgical procedures (especially to guide subsequent wire localization and excisional biopsy), and pathologic examination (particularly to indicate the tumor bed in the event neoadjuvant chemotherapy leads to apparent dissolution of the tumor mass). However, the use of larger needles and vacuum-assisted techniques removes relatively larger volume of tissue, leaving a large biopsy cavity. Marker clips placed in this setting have been reported to become displaced or “migrate” a distance of more than 1 cm from the biopsy site in 28% of cases.172 In an attempt to prevent migration, clips “with grips” and those surrounded by bioresorbable collagen “plug” material have been devised. The plug has a distinctive “collagen-like” histologic appearance, and it elicits only minimal reactive changes. However, microcalcifications formed in these plugs within 7 to 14 months can hinder subsequent radiologic follow-up (see later).
Changes in Number of Calcifications in Clinical Mammograms
Spontaneous “disappearance” of calcifications has been documented in vivo with serial mammograms.173,174 The composition of calcifications prone to this process is not known, but follow-up studies suggest that the associated lesions are usually benign.173 A study of NCB specimens revealed that radiographically detectable calcifications could no longer be seen in x-rays of the specimens after several days of storage in aqueous solutions, including 10% formalin, whereas the calcifications were preserved in samples stored in ethanol.175
An increase in the number and extent of calcifications raises concern for the presence of carcinoma. Rapid expansion of the area of calcifications has been associated with highgrade DCIS with necrosis (i.e., comedo DCIS), whereas slower growth of calcifications characterizes noncomedo DCIS.176 The mean doubling time for all DCIS based on the extent of calcifications was 118.0 ± 111.2 days (range, 18 to 539 days). The mean diameters of noncomedo and comedo DCIS based on the distribution of calcifications were 255.8 ± 71.1 µm and 302.7 ± 232.0 µm, respectively, using a computerized image analysis system.
Calcifications and Systemic Diseases
Mammographically detected calcifications can be a manifestation of systemic conditions.177 Awareness of various patterns of calcifications as well as other forms of radiologic abnormalities in various systemic diseases can aid in differentiating primary mammary lesions from systemic diseases. In one case, a diagnosis of Klippel-Trénaunay syndrome (characterized by nevus flammeus, vascular malformations, and hypertrophy of soft and bony tissue) was made after calcifications found in a routine mammogram were shown to be localized to subcutaneous vascular structures.178 Chronic renal failure can be associated with “metastatic” calcifications in soft tissues and blood vessels at various sites including the breast.179,180
The relationship of arterial calcifications to cardiovascular disease and diabetes mellitus has been the subject of a number of studies. Arterial calcifications were found in 9% of mammograms from women aged 50 to 68 years at entry into a screening program, and in 15.4% of the women studied who had diabetes mellitus.181 Overall excess cardiovascular mortality of 40% was found after 16 to 19 years of follow-up among women with arterial calcifications (hazard ratio [HR], 1.4; 95% CI, 1.1 to 1.8), and in the group with diabetes excess mortality due to cardiovascular disease was 90% (HR, 1.9; 95% CI, 1.1 to 3.2).
Maas et al.182 studied risk factors associated with mammographically detected arterial calcifications in a cohort derived from a breast cancer-screening program. Arterial calcifications were found in 194 of 1,699 women (11%). The frequency increased with age. After adjusting for age, the presence of arterial calcifications was associated with parity, being present in 2.5% of nulliparous women, 9% with one or two children, and in 17% of women with three or more children. Arterial calcifications were significantly related to breast-feeding. Maas et al.183 have also reported that the prevalence of arterial calcifications in mammograms from a cohort of women with increased risk of cardiovascular disease was 23%. The risk of arterial calcifications was 58% greater in diabetics than in nondiabetics. Kataoka et al.184 found that arterial calcifications detected in screening mammograms were associated with prevalent coronary artery disease, with an odds ratio of 2.54 (95% CI, 1.03 to 6.30). There was an inverse relation to smoking, an observation also reported by Maas et al.182 These and other studies185,186 suggest that mammographically detected vascular calcifications may be an indicator of coronary heart disease, especially in women with diabetes mellitus.
Noncalcified Crystalloids
Noncalcified crystalloids found in the salivary gland ducts also occur very rarely in mammary ducts or lobules, and
they may be associated with DCIS.187 These deposits are not visualized in mammograms and are not birefringent. Histologic examination reveals eosinophilic, variously shaped crystal-like deposits (needles, rhomboid, hexagonal, plate, and tetrahedral) measuring up to 500 µm with an average size of 20 µm (Fig. 44.13). Secretion on the surfaces of crystalloids is stained with Alcian blue, mucicarmine, periodic acid-Schiff (PAS), and the antibody for epithelial membrane antigen (EMA). Crystalloids are electron-dense when examined by electron microscopy, and they do not contain calcium phosphate hydroxyapatite or calcium oxalate. The source of crystalloids in the breast is not known, but it has been speculated that they are formed by crystallization of protein-carbohydrate complexes in abnormal secretions produced by the epithelium.
they may be associated with DCIS.187 These deposits are not visualized in mammograms and are not birefringent. Histologic examination reveals eosinophilic, variously shaped crystal-like deposits (needles, rhomboid, hexagonal, plate, and tetrahedral) measuring up to 500 µm with an average size of 20 µm (Fig. 44.13). Secretion on the surfaces of crystalloids is stained with Alcian blue, mucicarmine, periodic acid-Schiff (PAS), and the antibody for epithelial membrane antigen (EMA). Crystalloids are electron-dense when examined by electron microscopy, and they do not contain calcium phosphate hydroxyapatite or calcium oxalate. The source of crystalloids in the breast is not known, but it has been speculated that they are formed by crystallization of protein-carbohydrate complexes in abnormal secretions produced by the epithelium.
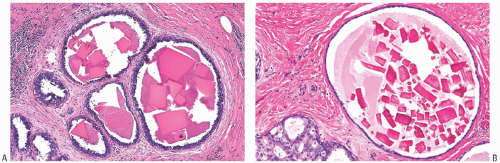 FIG. 44.13. Noncalcified crystalloids. A,B: Characteristic rectangular and triangular shapes are shown associated with proteinaceous secretion in small cysts. B: A gland contiguous to one with crystalloids shows ADH. |
Liesegang-like rings, concentric structures formed by a complex chemical reaction, can occasionally be encountered in benign mammary glands with pregnancy-like changes.188 These rings may not be mammographically evident, unless there is a calcified center therein.
NCB AS AN INITIAL DIAGNOSTIC PROCEDURE
The increasing use of ultrasound-directed or stereotacticguided core biopsies has made it possible to reliably sample a growing number of nonpalpable radiographically detected lesions. In one series of 100 such lesions at least 5 mm in diameter, NCB procedures correctly identified 35 of 36 carcinomas that were confirmed by surgical biopsy.189 Five to six core biopsy specimens obtained with a 14-G needle provide adequate samples for the diagnosis of most lesions. Liberman et al.190 found that six cores proved to be diagnostic in 92% of mammographic lesions with calcifications, whereas five cores were sufficient for 99% of mass lesions.
 FIG. 44.14. Displaced carcinoma in a stereotactic needle core biopsy sample. This specimen was mistakenly interpreted as invasive carcinoma. It shows carcinoma displaced in fat. The subsequent excisional biopsy showed only DCIS with additional displaced epithelium. |
There is generally a high level of concordance between the diagnosis obtained with stereotactic NCB and the subsequent surgical excision. Gisvold et al.191 reported a slightly higher concordance rate for benign (90%) than for malignant lesions (85%) if at least five cores were obtained. When there were fewer than five cores, the concordance rates decreased to 66% and 34% for benign and carcinomatous lesions, respectively. The positive predictive value (PPV) of stereotactic NCB for the presence of invasion in patients with carcinoma was 98% in 48 cases.192 In the one discordant instance, a fragment of DCIS displaced into fat in a core biopsy sample was mistakenly interpreted as invasive carcinoma (Fig. 44.14). Intrinsic invasive carcinoma was found in 3 of 15 excisional biopsy specimens from other patients who had only DCIS in needle biopsy samples. Liberman193 has reviewed the diagnostic reliability of NCB procedures. A high level of concordance
between tumor type and grade, as well as hormone receptors and HER2 status between NCB and the subsequent surgical excision, is generally obtained.
between tumor type and grade, as well as hormone receptors and HER2 status between NCB and the subsequent surgical excision, is generally obtained.
Pathologic Changes Attributable to Needling Procedures
The placement of localizing wires has become precise with the widespread use of stereotactic procedures. Direct penetration of the lesion is sought when biopsy is combined with localization. Follow-up mammography performed 6 months after stereotactic 14-G biopsy revealed no mammographically detectable architectural distortion attributable to the procedure in 24 patients studied by Kaye et al.194 In two instances, there were fewer calcifications in postbiopsy mammograms, and a 6-mm fibroadenoma contained a 3 × 2 mm2 defect. These and other observations suggest that the procedure, at least when performed conservatively, usually does not cause longstanding structural changes in the breast that might interfere with later radiographic studies.
Nonetheless, some traumatic changes invariably occur in the breast tissue as a result of needle localization and biopsy procedures (Figs. 44.14, 44.15, 44.16, 44.17, 44.18, 44.19, 44.20, 44.21, 44.22, 44.23, 44.24, 44.25 to 44.26). Displacement of benign or malignant epithelium into the breast stroma can be found in subsequent excisional biopsies. Fragments of cutaneous squamous epithelium displaced by needles into the breast can form epidermal inclusion cysts.195 Conversely, rare instances of carcinoma displaced to the skin as a result of NCB procedures have been reported. In one instance, carcinoma cells accompanied by hemosiderin-laden macrophages were found in dermal lymphatic channels after an NCB, and mastectomy revealed DCIS with carcinomatous epithelial displacement in the needle track and in lymphatics of the breast.196 Carcinomatous deposits in the dermis of the skin after “multiple-puncture biopsy” were reported in two patients by Stolier et al.197 One of these patients developed a clinical skin recurrence 34 months after the procedure. Chao et al.198 described two patients with subcutaneous recurrence of carcinoma in the site of a prior NCB procedure. The recurrences were detected 12 and 17 months postbiopsy. A third patient was found to have microscopic deposit of carcinoma in the skin in a mastectomy specimen.
Displaced DCIS cells have been observed in breast stroma and within vascular channels in breast specimens obtained subsequent to needling procedures. In patients with DCIS, these displaced carcinoma fragments can mimic stromal invasion, and, in some circumstances, they represent a potential source of misdiagnosis of intrinsic invasive carcinoma. Histologic findings suggesting displacement of DCIS include the presence of scattered, isolated fragments of carcinomatous epithelium in artifactual spaces within breast stroma, accompanied by hemorrhage, fat necrosis, inflammation, hemosiderin-laden macrophages, or granulation tissue. Calcifications and “foamy” histiocytes of the type commonly seen in intraductal lesions may be associated with displaced carcinomatous epithelium in the stroma or in vascular channels. The extent to which breast needling procedures might contribute lymphovascular dispersal of tumor cells has not been determined.
Papillary lesions are especially vulnerable to epithelial displacement after needling procedures because of their intrinsic fragile structure.199,200 Reactive changes at needling sites in papillary lesions may evolve into nodular foci of spindle cell proliferation that are composed of proliferating myofibroblasts and blood vessels, as well as inflammatory cells.201 Benign and carcinomatous papillary epithelium may be found embedded in such nodules.
Boppana et al.202 reviewed 100 consecutive breast carcinomas that had been subjected to prior FNA, and noted displaced epithelial fragments in 36% of cases. Youngson et al.203,204 reviewed slides from 43 consecutive cases in which surgical biopsy and/or mastectomy had been performed following an initial 14-G core biopsy diagnosis of breast carcinoma, and identified displaced epithelial fragments outside of the primary lesion in 12 of the cases (28%). However, multiple needling procedures (e.g., local anesthetic injection in all 43 cases, needle localization in 22 of 43 cases, suture placement in 18 of 43 cases, and FNA in 1 of 43 cases) had been performed in each of the cases reviewed. Hoorntje et al.205 reported finding displaced carcinoma cells in 11 of 22 (50%) needle tracks after 14-G needle biopsy procedures. Prospectively, these authors found displaced carcinoma in 7 (64%) or 11 needle tracks examined 7 to 35 days (median interval, 25 days) after 14-G needle biopsy. The frequency of epithelial displacement in the needle track has been reduced substantially but not eliminated since the introduction of vacuum-assisted stereotactic biopsy with an 11-G needle.193
The clinical significance of epithelial displacement remains largely uncertain. Chen et al.206 investigated the potential role of NCB as a factor predisposing to breast recurrence after conserving surgery with radiotherapy. The breast recurrence rate was lower (2.3%) in women who underwent excision after a diagnostic NCB procedure than in those who had a needle-localized excisional biopsy without prior core
biopsy (5.4%) and patients with palpable tumors who had a nonimaged guided excision (10.3%). Although other factors such as margin status and tumor size probably influenced these results, they suggest that epithelial displacement that occurs in a substantial number of NCB sites is not a major factor contributing to breast recurrence. In many instances, the potential risk of local recurrence posed by displaced carcinoma is ameliorated because the biopsy site and needle track are removed in the subsequent excision.
biopsy (5.4%) and patients with palpable tumors who had a nonimaged guided excision (10.3%). Although other factors such as margin status and tumor size probably influenced these results, they suggest that epithelial displacement that occurs in a substantial number of NCB sites is not a major factor contributing to breast recurrence. In many instances, the potential risk of local recurrence posed by displaced carcinoma is ameliorated because the biopsy site and needle track are removed in the subsequent excision.
Epithelial displacement presents a challenging diagnostic histopathologic problem. Eliciting a history of a previous needling procedure may help prevent an erroneous diagnosis of invasive carcinoma. A diagnosis of carcinoma should not be based solely on the presence of epithelium within stroma, since epithelial displacement has been observed following needling procedures in benign breast lesions, notably papillary ductal hyperplasia and intraductal papilloma.
 FIG. 44.17. Displaced apocrine epithelium and skin. Fragments of apocrine epithelium and epidermis from the skin have been displaced into the lumen of a duct. |
The significance of carcinomatous lymphovascular emboli in the setting of epithelial displacement remains uncertain. Until further clinical information becomes available, the finding of lymphatic tumor emboli is considered to be a risk factor for transport of carcinoma cells to axillary lymph nodes (ALNs), even when conventional stromal invasion cannot be identified. As a general principle, the presence of carcinoma cells in a lymph node capsule or subcapsular sinus should be considered to represent metastatic carcinoma, even when no intrinsic stromal invasion is found in the breast. These cases often have carcinoma cells in lymphovascular spaces in the breast parenchyma in the vicinity of the needling procedure. One unusual patient presented with a clinically enlarged ALN diffusely involved by metastatic carcinoma 7 years after a mastectomy for DCIS that had been diagnosed by needle biopsy. Lymph nodes removed from the axilla at the time of mastectomy had no carcinoma identified in routine sections. Upon review, the site of the NCB in the breast exhibited carcinoma cells in lymphatic channels. The viability of DCIS or invasive carcinoma cells that may have been traumatically displaced into lymphovascular channels and possibly deposited in sites outside the breast is unknown, but the case just described raises the possibility that such cells could persist for a substantial period.
THERMAL (ELECTROCAUTERY) DAMAGE IN BIOPSY SPECIMENS
Electrocautery instruments used for the excision of tissue from the breast and other sites decrease blood loss from dissected tissues because the surfaces are coagulated as they are separated by the cutting edge. When used to perform an excisional breast biopsy, these instruments reduce the risk of hematoma formation in the biopsy cavity, and the operation can be completed in a shorter period of time.207
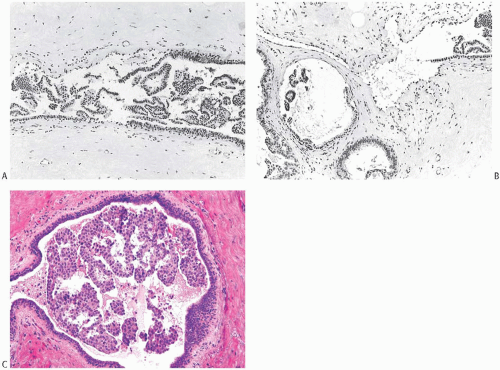 FIG. 44.18. Displaced epithelium within ducts. These photographs illustrate ductal epithelium displaced by FNA procedures. A: Fragments of ductal epithelium that were stripped off the basement membrane have been displaced into the ductal lumen. The detached epithelium resembles a papillary proliferation. B: Part of the duct shown in (A) is present in the upper right corner. There is detached epithelium in the cyst on the left. C: Papillary fragments of apocrine carcinoma are shown displaced into the lumen of an inactive duct after an FNA procedure. |
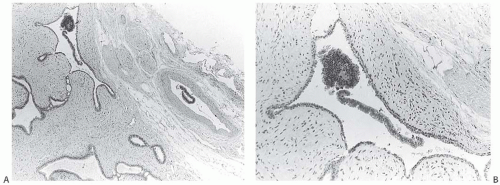 FIG. 44.19. Displaced benign epithelium in a fibroadenoma. A: Detached epithelium in an epithelial cleft in the tumor and in an artery on the right after an FNA biopsy. B: Epithelium in the cleft. C: Benign epithelium in the artery. |
The thermal effect may produce significant changes in the tissues. Reduced biochemically measured estrogen receptor (ER) activity had been described some years ago when such testing was the norm,208,209,210 with the decrease in receptor activity sufficient to result in a false-negative report.211 Immunohistochemical study of breast (and prostate) specimens after electrocautery treatment also reveals a decrease in steroid binding208,209(Fig. 44.27).
The histologic changes in the breast caused by thermal injury are similar to those seen at other sites. Thermal damage is most severe at the surface of the specimen, generally penetrating not more than 1 mm. The manner in which the instrument is used, the character of the tissue, and the size of the specimen removed influence the intensity and depth of the effect. The most significant effects are alterations in cytologic detail. Microscopic architecture may be so distorted that the distinction between normal, hyperplastic, and neoplastic tissues is impossible (Figs. 44.28 and 44.29). Because the damage is maximal at the edges or surfaces of the tissues, thermal artifacts can severely limit the assessment of the margins of excision (Figs. 44.28 and 44.30). If an excision is carried out with little or no breast parenchyma surrounding a carcinoma, thermal damage may occur in the tumor itself. In addition to the risk of altering results of receptor activity testing, the histologic artifacts can interfere with the diagnosis or classification of the tumor, and with the assessment of microscopic features of the tumor such as histologic grade (Figs. 44.29, 44.31, and 44.32). Thermal artifacts may make it impossible to determine whether DCIS is present in tissue outside the tumor mass. It is possible to encounter rare instances in which cautery-induced changes are so egregious throughout an excisional biopsy specimen that no diagnosis could be rendered and the presence of carcinoma could not be affirmed or refuted (Figs. 44.28 and 44.29). In this situation, the patient may undergo reexcision to determine whether carcinoma remains at the biopsy site. Since the features of the primary tumor are critical for assessing prognosis and determining therapy, such a patient is left not knowing whether she had carcinoma, what her prognosis might be, and how she should be treated. Fortunately, this scenario is infrequent, but it serves as a reminder that the primary purpose of a surgical biopsy is to obtain a specimen for histologic diagnosis, and that the procedure should be performed in a manner most likely to achieve this goal.
The use of ultrasonic dissecting instruments that enable safe (if slower) dissection and better hemostasis, as well as less artifact, has gained limited acceptance in breast surgery. Ultrasonic dissection utilizes high-frequency vibrations within the “harmonic” frequency range to cut and coagulate tissues. It induces lesser thermal artifact because of lower operating temperature than electrocautery.212,213
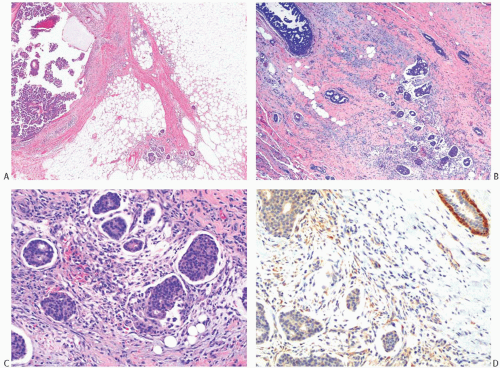 FIG. 44.21. Displaced papillary carcinoma. A: Intracystic papillary carcinoma (left), status-post NCB, with seeding of the needle track (right) in a healing biopsy site with fat necrosis. Images (B-D) are from a case of a noninvasive micropapillary carcinoma, status-post NCB. B: DCIS (upper left) and displaced DCIS in the needle biopsy track (right). C: Magnified view of the displaced DCIS. D: The smooth muscle actin immunostain shows no evidence of myoepithelial cells around the displaced clusters of carcinoma cells. Myoepithelial reactivity is evident in a benign duct (upper right). Myofibroblasts in the stroma around the displaced carcinoma are also immunoreactive. In some cases, myoepithelial cells may be detected by appropriate immunostains in clusters of displaced DCIS cells. |
THE MASTECTOMY SPECIMEN
Multiple variations of the mastectomy procedure can be performed—including those of the radical, modified radical, simple, nipple-sparing, skin-sparing, and partial types. In general, mastectomy has evolved from the taking of larger specimens to smaller specimens, that is, from one considered as a “mutilation” to an “aid to breast reconstruction.”214 The surgeon should specify the type of mastectomy performed in each instance, since this information cannot always be ascertained from gross examination of the specimen.
The purpose of the gross description of a mastectomy specimen is to document the extent of the operation and the characteristics of the tissue removed. A standard radical mastectomy can usually be oriented on the basis of landmarks in the specimen, especially the position of the muscle segments. This may not be so easily accomplished with various types of modified radical mastectomies that have almost replaced the radical mastectomy. It is important that the surgeon indicate if there is anything unusual about the specimen and that important landmarks such as levels of ALNs be identified.
The external description of the specimen should include the following: overall size, dimensions and appearance of the skin with measurement of scars or incisions, appearance of the nipple and areola (if present), presence of muscle and
axillary tissue, and location of any distinct palpable lesion. There is an increasing trend to take a minimal central portion of the skin of the breast or to remove no skin with the mastectomy specimens.
axillary tissue, and location of any distinct palpable lesion. There is an increasing trend to take a minimal central portion of the skin of the breast or to remove no skin with the mastectomy specimens.
Dissection of the specimen is easily accomplished by placing it skin-side down anatomically oriented in order to identify the quadrants. One visualizes the findings as if one were looking through the patient from back to front. The external noncutaneous surfaces should be inspected and palpated for evidence of tumor involvement and inked. For a standard radical mastectomy, the cut edge of the sternal attachment of the pectoralis major muscle marks the medial side. In such a specimen, the fascia between the major and minor pectoralis muscles should be dissected to identify interpectoral (Rotter) lymph nodes.
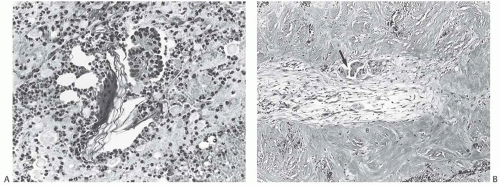 FIG. 44.23. Displaced intraductal carcinoma in a needle track. A: Detached fragments of papillary carcinoma and squamous epithelium (presumably from the skin) are shown surrounded by inflammatory cells in a needle track. B: Detached fragments of DCIS (arrow) are shown in granulation tissue at the site of a healing needle track. |
The breast is sectioned by a series of parallel incisions at least 5 mm apart through the posterior surface up to the skin. A tumor, if present, should be described in the same fashion as for a biopsy specimen. The size and character of a biopsy site should be noted, including areas of induration. It is preferable that these fibrotic areas not be identified as tumor, since the reaction in a healing biopsy cavity may be grossly indistinguishable from carcinoma. The appearance of the remaining breast parenchyma is also recorded, including relative proportions of fat and fibrous parenchyma, the size, location and character of any discrete lesions, and the presence or absence
of cysts. It is generally recommended that samples for histologic examination be taken from the tumor and/or biopsy site, nipple, skin, quadrants, and the margins, including the deep surface under the tumor as well as the superficial surface not covered by skin. However, the value of random sampling was questioned by Sikand et al.,215 who studied more than 200 consecutive mastectomy specimens. Multifocality was found in 21% of breasts with invasive carcinoma but in none of 34 breasts with DCIS. Overall, when Paget disease of the nipple and carcinoma in the quadrants were considered, multifocality was found in 7% of mastectomies. The authors concluded that careful gross examination would detect about 50% of multifocal carcinoma and that the information was rarely clinically important. United Kingdom’s National Health Service Breast Screening Programme guidelines indicate that routine sampling of the nipple and quadrants is not mandatory and should be done if “resources permit.”216 However, sampling of the grossly unremarkable nipple and quadrants is recommended in the United States since samples from these sections may reveal unsuspected invasive carcinoma.
of cysts. It is generally recommended that samples for histologic examination be taken from the tumor and/or biopsy site, nipple, skin, quadrants, and the margins, including the deep surface under the tumor as well as the superficial surface not covered by skin. However, the value of random sampling was questioned by Sikand et al.,215 who studied more than 200 consecutive mastectomy specimens. Multifocality was found in 21% of breasts with invasive carcinoma but in none of 34 breasts with DCIS. Overall, when Paget disease of the nipple and carcinoma in the quadrants were considered, multifocality was found in 7% of mastectomies. The authors concluded that careful gross examination would detect about 50% of multifocal carcinoma and that the information was rarely clinically important. United Kingdom’s National Health Service Breast Screening Programme guidelines indicate that routine sampling of the nipple and quadrants is not mandatory and should be done if “resources permit.”216 However, sampling of the grossly unremarkable nipple and quadrants is recommended in the United States since samples from these sections may reveal unsuspected invasive carcinoma.
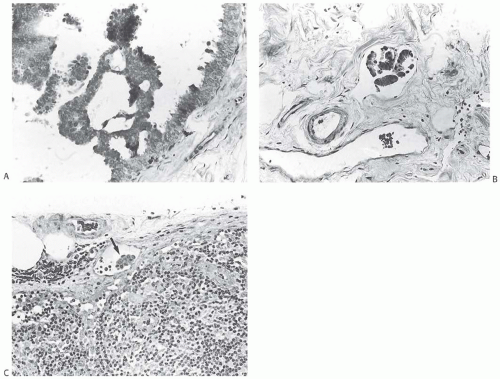 FIG. 44.24. Displaced intraductal carcinoma in lymphatic spaces of the breast and carcinoma in axillary lymph nodes. The patient had an FNA biopsy and stereotactic localization before this excisional biopsy was performed. A: Micropapillary DCIS. B: Clusters of DCIS cells in a lymphatic channel. C: An axillary dissection performed after intralymphatic carcinoma was found in the breast biopsy revealed small clusters of carcinoma cells in the capsular lymphatics of lymph nodes such as the one shown here (arrow) and in peripheral sinusoids. |
A vertical section of the nipple will usually suffice to detect Paget disease or direct involvement of the nipple by carcinoma, even when it is not clinically suspected. With more elaborate sectioning of the nipple, unsuspected foci of carcinoma may be detected, but these are generally Paget disease or DCIS in lactiferous ducts that are of negligible clinical significance for a patient treated by mastectomy. Hence, preparing multiple sections of the nipple is not cost-effective for routine use. Nipple-sparing mastectomies are generally not indicated in tumors that are centrally located.217 FSE of the nipple margin is a useful procedure, and can help ensure a low recurrence rate following a nipple-sparing mastectomy.218,219
CLINICAL STAGING OF THE ALNS
Optimal staging of the axilla requires histopathologic examination of at least 10 lymph nodes.220 Typically, a dissection of axillary level I and II lymph nodes should yield about 15 lymph nodes, and one including level III should approach 20 lymph nodes. Some aspects of the pathology of ALNs are discussed in Chapter 43. SLN biopsy is discussed in a separate section later in this chapter.
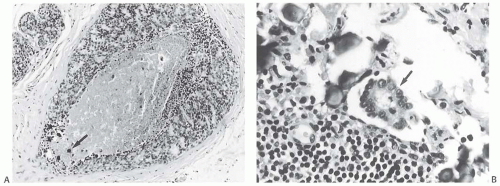 FIG. 44.25. Displaced intraductal carcinoma in the breast and carcinoma in axillary lymph nodes. A: Comedo DCIS in an excisional biopsy specimen after a stereotactic NCB and needle localization for nonpalpable mammographically detected calcifications. Small granular calcifications are present in the DCIS (arrow). B: A cluster of carcinoma cells (arrow) and several calcifications in the peripheral sinus of an ALN obtained after the procedure that yielded the DCIS shown in (A). |
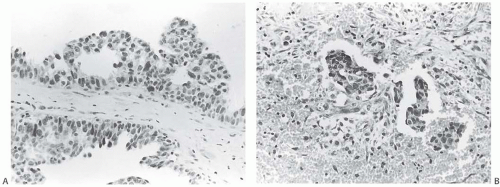 FIG. 44.26. Displaced intraductal carcinoma in the breast and a subsequent axillary lymph node metastasis. A: In 1987, this patient had an FNA biopsy obtained for mammographically detected calcifications. An excisional biopsy performed shortly thereafter revealed DCIS of the papillary and cribriform types shown here. B,C: Fragments of displaced DCIS were present in the biopsy specimen. D: These clusters of displaced carcinoma cells were present in granulation tissue at the excisional biopsy site in the subsequent mastectomy specimen. No intrinsic invasive carcinoma was found in the breast, and no metastatic carcinoma was identified in eight ALNs included with the 1987 mastectomy specimen. E: In 1994, the patient presented with an ipsilateral axillary tumor that proved to be metastatic mammary carcinoma in a lymph node. The growth pattern duplicated that of the previous DCIS. A likely explanation for this set of circumstances is that DCIS displaced to the lymph node in 1987 survived and, in the intervening 7 years, progressed to invasive carcinoma. No carcinoma was detected in the contralateral breast. |
 FIG. 44.27. Cautery effect and estrogen receptors. This biopsy sample shows nuclear reactivity in intact carcinoma on the right. No staining is present in cauterized carcinoma on the left. |
Nonsurgical Imaging Methods for Staging of the ALNs
Clinical methods for detecting metastatic carcinoma in ALNs preoperatively have been in development for several years, but have thus far not been sufficiently effective to be used routinely.221 These procedures have involved injection of radioactive tracers such as technetium Tc 99m,222,223,224,225 computed tomography (CT),226,227 MRI,228,229,230 positron emission tomography (PET) with radiolabeled glucose analogs,231,232 and sonography.233,234
Yoshimura et al.229 used MRI to evaluate ALNs preoperatively in 202 patients. They were able to detect lymph nodes in 200 of the patients (99%). When compared with clinical assessment, MRI was more accurate (88% vs. 79%) in predicting axillary nodal status. Computer programs have been
developed to select lymph node regions of interest (ROI) for automated screening of MR images, and in one study the maximum enhancement ratio of automated ROI was a strong predictor of axillary nodal status.230
developed to select lymph node regions of interest (ROI) for automated screening of MR images, and in one study the maximum enhancement ratio of automated ROI was a strong predictor of axillary nodal status.230
CT scanning has been compared with SLN biopsy as a method for staging the axilla. Miyauchi et al.226 studied 51 women who had a full axillary dissection after CT scanning and SLN biopsy. False-negative SLN biopsy occurred in 3 of
51 cases (6%) when non-SLNs (NSLNs) contained metastatic carcinoma after the SLN biopsy was negative. False-negative CT interpretations were recorded in 10 cases (19.7%). CT scans were able to identify the SLN in 42 of 51 cases (82.4%). Hata et al.227 investigated thin-section CT as a method for improving the detection of ALN metastases and reported 93.8% sensitivity for detecting positive lymph nodes. However, specificity (detection of node-negative cases) remained relatively low (82.1%) because the procedure was unable to detect micrometastases.
51 cases (6%) when non-SLNs (NSLNs) contained metastatic carcinoma after the SLN biopsy was negative. False-negative CT interpretations were recorded in 10 cases (19.7%). CT scans were able to identify the SLN in 42 of 51 cases (82.4%). Hata et al.227 investigated thin-section CT as a method for improving the detection of ALN metastases and reported 93.8% sensitivity for detecting positive lymph nodes. However, specificity (detection of node-negative cases) remained relatively low (82.1%) because the procedure was unable to detect micrometastases.
PET scanning detected 19 of 24 patients (79%) with histologically documented lymph node metastases in a series of 51 women studied after injection of 2-deoxy-2-fluoro[18F]-d-glucose (FDG).231 In this series, PET imaging was 96% correct in identifying women without axillary nodal metastases. Among women with tumors larger than 2 cm, PET accurately identified 94% of women with nodal metastases (sensitivity) and 100% with negative lymph nodes (specificity). In the pathologic T1 (pT1) group with tumors smaller than 2 cm, PET had a sensitivity of only 33%. The authors concluded that PET imaging was not a “substitute for histopathologic analysis in detecting ALN metastases.”
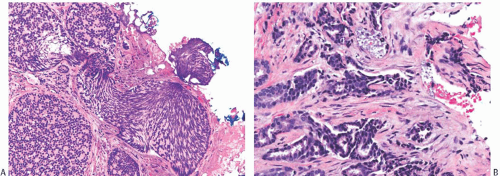 FIG. 44.30. Cautery effect. A: The tissue is distorted by cautery effect at the margin. This area can be reasonably interpreted as showing DCIS at the margin because it is continuous with underlying carcinoma. B: This isolated distorted area at the edge of another specimen can, in the appropriate context, be reasonably interpreted as invasive ductal carcinoma. |
A prospective multicenter study of axillary staging by PET imaging obtained satisfactory data from 308 of 360 enrolled women (86%).232 The positive and negative predictive values were 62% (60% to 64%) and 79% (76% to 81%), respectively.
Sonography has also been investigated as a method for staging ALNs. Alvarez et al.233 reviewed 16 published reports and concluded that “axillary sonography is moderately sensitive and fairly specific.” When lymph node size was used as the diagnostic criterion in women with clinically negative axillae, sensitivity in various reports ranged from 48.8% to 87.1% and specificity from 55.6% to 97.3%. Using lymph node morphology, sensitivity ranged from 26.4% to 75.9% and specificity from 88.4% to 98.1%. Ultrasound-guided core biopsy and FNA cytology are useful procedures for confirming the presence of metastatic carcinoma in lymph nodes.233,234 FNA




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree