Pathologic Effects of Therapy
ELENA BRACHTEL
FREDERICK C. KOERNER
RADIATION
Radiation and Hodgkin Lymphoma
The breasts may be exposed secondarily to radiation during multiple diagnostic procedures such as mammography and fluoroscopy,1 or in the course of radiotherapy administered to another organ, such as mediastinal radiotherapy for Hodgkin lymphoma.2,3,4,5 The radiation exposure in these situations has been associated with an increased risk of the subsequent development of breast carcinoma.2,6,7,8,9 Wendland et al.10 reported that the standard incidence ratio (SIR) of breast carcinoma among Hodgkin lymphoma patients who received radiotherapy was 3.17, with a 95% confidence interval (CI) of 2.66 to 3.79 when compared to the general population. The SIR of breast carcinoma in irradiated female Hodgkin lymphoma patients compared to nonirradiated patients was 1.90. In the same study, the SIR of breast carcinoma in nonradiated Hodgkin lymphoma patients when compared to the general population was also elevated (1.67; 95% CI, 1.24 to 2.20). Each of these differences was statistically significant, indicating that women treated for Hodgkin lymphoma have an elevated risk of breast carcinoma that is enhanced in irradiated women.
In one study, the relative risk (RR) of developing breast carcinoma in women irradiated for Hodgkin lymphoma before the age of 15 years was 136 (95% CI, 34 to 371) compared to the general population, whereas among those treated at ages 15 to 19, 20 to 24, and 25 to 29 years, the RRs were 19, 19, and 7.3, respectively.11 Swerdlow et al.9 reported a very high SIR of breast carcinoma in patients treated at the age of 14 years (47.2), with 95% CI of 28.0 to 79.8. These results suggest greater susceptibility to breast carcinoma when radiation exposure is near puberty. Others reported the cumulative probability of developing breast carcinoma to be 35% by age 40 (95% CI, 17.4 to 52.6).3 Another study of women with Hodgkin lymphoma treated before age 21 found an RR of 26.2 (95% CI, 15.0 to 42.6) with a 20-year actuarial risk of 9.2%.12
Estimates of radiation dosage to the breasts during the treatment of Hodgkin lymphoma indicate that the exposure is at a potentially carcinogenic level.13,14 Kowalski and Smith15 studied the distribution of radiation with an anthropomorphic phantom. Some regions of the breast in the treatment fields had more than 70% of the prescribed dose, whereas blocked regions received 2% to 29% of the prescribed dose. Newer approaches to irradiation for Hodgkin lymphoma with mediastinal involvement utilize lower doses than the traditional mantle field irradiation (35 to 45 Gy) and more targeted irradiation. These techniques result in reduced exposure of other organs.16,17
Most studies report breast carcinoma after irradiation for Hodgkin lymphoma in women, but in rare instances breast carcinoma may also occur in men. The first author has studied one case in which breast carcinoma developed in a 55-year-old man who had undergone irradiation for Hodgkin lymphoma at the age of 12 years. His mother also developed carcinoma of the breast at the age of 50 years, and other members of the family suffered from carcinomas of other organs.
Data from the studies by Cutuli et al.18,19,20 included 189 patients who developed a total of 214 breast carcinomas after radiotherapy for Hodgkin lymphoma. The median age at diagnosis of Hodgkin lymphoma was 25 years. The median age at diagnosis of breast carcinoma was 42 years, with a median interval of 18.6 years. The frequency of bilaterality was 13.2%; most contralateral tumors were metachronous. Axillary lymph node (ALN) metastases occurred in 32% of the invasive carcinomas for which axillary dissection was performed.
Patients who received supradiaphragmatic radiotherapy for the treatment of Hodgkin lymphoma are candidates for surveillance for the early detection of breast carcinoma. Mammography is reported to have high sensitivity for detecting carcinomas in the breast of women irradiated for Hodgkin lymphoma, especially when calcifications are present.21,22,23,24 Ultrasonography may be employed as an adjunct to mammography, but one cannot rely on this technique as a primary screening modality because of a relatively high frequency of false-positive findings.25 Magnetic resonance imaging (MRI) is effective for detecting tumor-forming, largely invasive carcinomas in women with genetic and other high-risk predispositions to breast carcinoma, but it has less sensitivity than mammography for detecting ductal carcinoma in situ (DCIS).26,27 On this basis, MRI has been shown to be useful for screening irradiated Hodgkin lymphoma patients.28
No structural changes attributable to the level of radiation exposure after treatment for Hodgkin lymphoma are evident
when the mammary glandular tissue is examined histopathologically. Except for a trend to less desmoplastic reaction in carcinomas that arose in irradiated women, Dvoretsky et al.29 found no histologic difference between tumors in women previously irradiated for postpartum mastitis and a control group. Carcinomas in patients treated for Hodgkin lymphoma tend to be poorly differentiated, but in other respects they are not significantly different pathologically from tumors in women without prior irradiation.5,30 Nearly all carcinomas have been ductal, with very rare examples of infiltrating lobular and special types such as mucinous carcinoma.19,20 Approximately 15% of the carcinomas are DCIS. The carcinomas are more likely to be bilateral, and both synchronous and metachronous bilateral carcinomas have been reported.20,23,31
when the mammary glandular tissue is examined histopathologically. Except for a trend to less desmoplastic reaction in carcinomas that arose in irradiated women, Dvoretsky et al.29 found no histologic difference between tumors in women previously irradiated for postpartum mastitis and a control group. Carcinomas in patients treated for Hodgkin lymphoma tend to be poorly differentiated, but in other respects they are not significantly different pathologically from tumors in women without prior irradiation.5,30 Nearly all carcinomas have been ductal, with very rare examples of infiltrating lobular and special types such as mucinous carcinoma.19,20 Approximately 15% of the carcinomas are DCIS. The carcinomas are more likely to be bilateral, and both synchronous and metachronous bilateral carcinomas have been reported.20,23,31
Mastectomy is still performed more often than lumpectomy for the primary surgical treatment of breast carcinoma that develops after Hodgkin lymphoma treated with radiotherapy, although these breast carcinomas are now more likely to be detected at a lower stage.30,32 Deutsch et al.33 described 12 patients successfully treated with lumpectomy and radiation with “good to excellent cosmetic results” and “no significant acute adverse reactions and no late sequelae” after a median follow-up of 46 months.
Radiation and Breast Conservation Therapy
Radiation of the breast as a component of breast conservation treatment for mammary carcinoma is usually performed after surgery to decrease the risk of ipsilateral recurrence.34 Conventional irradiation protocols involve levels of exposure of 45 to 50 Gy often with a boost of 10 to 16 Gy to the site of the excision.35 In selected cases of early-stage breast carcinoma, partial breast irradiation (PBI) is currently performed in some centers for selected patients who meet the eligibility recommendations set out by the American Society for Radiation Oncology.36,37
Long-term clinical effects of therapeutic mammary radiation on the normal breast evolve over a period of months to years,38 and the extent to which these occur varies among individuals. Some women ultimately exhibit diffuse increased firmness or sclerosis of the breast, but in the majority this is mild and the tissue remains elastic. Ptosis, a natural change of aging, is less pronounced in the irradiated breast. Cutaneous atrophy and telangiectasia are likely to be more conspicuous in areas that received a radiation boost.38,39 The irradiated breast is usually unable to lactate but the untreated breast is unaffected.40,41 Radiation can be delivered effectively to the breast containing an implant, although there may be a tendency for more frequent fibrous encapsulation of the prosthesis or for infection to occur.42,43 The adverse clinical effects of radiation may be more severe in patients who have a collagen vascular disease such as scleroderma, systemic lupus erythematosus, or other related conditions.44,45
After breast-conserving treatment for breast carcinoma, patients are screened following national guidelines for ipsi- or contralateral recurrence. Imaging of the operated and irradiated breast poses challenges and is considered less sensitive for the detection of abnormalities than imaging of the the untreated breast. However, common benign post-treatment changes such as fat necrosis, parenchymal distortion, and scarring often can be distinguished from recurrence radiographically.46,47 MRI studies, in which focal enhancement of the treated breast is expected, are of limited use in the immediate post-treatment period. This modality shows high sensitivity and specificity when used to distinguish scar tissue from tumor recurrence at least 12 months after the last intervention in the breast.47,48,49
Coarse, scattered benign-appearing calcifications may be found in 25% of irradiated breasts, and these are generally of little concern.50 On the other hand, new, clustered pleomorphic calcifications present a significant problem.51 Calcified sutures in the biopsy site may mimic the pattern of calcifications associated with carcinoma, although rarely suture calcifications have a distinctive knotted configuration.52 Fat necrosis, which often has a stellate configuration and may contain calcifications, is often recognized as such radiologically.53,54 Localized, encapsulated fat necrosis with a cystic appearance has been described at the site of iridium implantation50 (Fig. 41.1). Other forms of brachytherapy or intraoperative radiation are also associated with increased fat necrosis.55,56 Occasionally, fat necrosis causes an enhancing lesion on MRI in the area of the scar that is not easily distinguishable from recurrent tumor.
Cases exhibiting a new mass, a suspicious area of enhancement on MRI, a change in an existing postoperative scar, or the appearance of new, pleomorphic calcifications warrant a biopsy to exclude recurrence of the carcinoma. Early detection of a local recurrence has been shown to be associated with improved long-term outcome.57 Recurrences that may present as invasive or in situ carcinoma most often occur
2 to 6 years after completion of breast-conserving treatment and are typically located close to the site of prior operation. Carcinomas that develop more than 10 years following treatment and those that arise distant from the scar more likely represent new primary carcinomas.46
2 to 6 years after completion of breast-conserving treatment and are typically located close to the site of prior operation. Carcinomas that develop more than 10 years following treatment and those that arise distant from the scar more likely represent new primary carcinomas.46
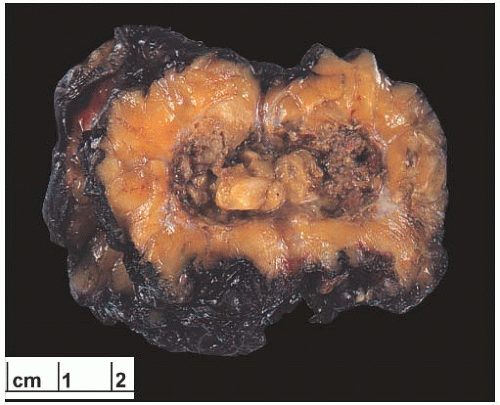 FIG. 41.1. Radiation-induced fat necrosis. Cystic fat necrosis at the site of prior interstitial and external beam radiation therapy after lumpectomy for invasive ductal carcinoma. |
The risk of second malignant neoplasms that develop after radiotherapy as part of breast conservation therapy was studied by Obedian et al.58 The authors compared the outcomes of 1,029 patients treated by lumpectomy with radiation (LRT) and 1,387 patients treated by mastectomy (MAST). The median follow-up was 14.6 and 16 years, respectively. The risk of contralateral carcinoma was 10% in both cohorts. For women 45 years or younger at the time of treatment, the risks of contralateral carcinoma were not significantly different (LRT, 10%; MAST, 7%) after 15 years of followup. The frequency of contralateral carcinoma was lower in women who received adjuvant hormonal therapy, but the difference was not statistically significant. During an interval of 15 years, nonmammary malignant neoplasms were diagnosed in 11% of LRT patients and 10% of MAST patients. The risk of lung carcinoma was higher among smokers than among nonsmokers who received radiotherapy (p = 0.06). Radiotherapy for breast carcinoma has also been associated with a significantly increased RR of carcinoma of the esophagus and lung.59,60 A small increased risk of acute myeloid leukemia after locoregional radiation for breast carcinoma was enhanced by chemotherapy.61 However, the overall incidence of such neoplasms appears to be low, with about 160 cases observed in a cohort of 33,763 women with breast carcinoma who had radiotherapy.59 The relationship of breast irradiation to the development of angiosarcoma is discussed in Chapter 39.
Microscopic Pathology
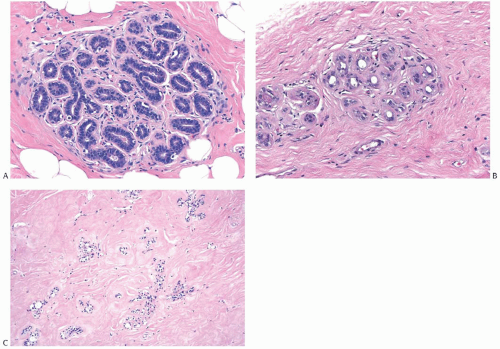
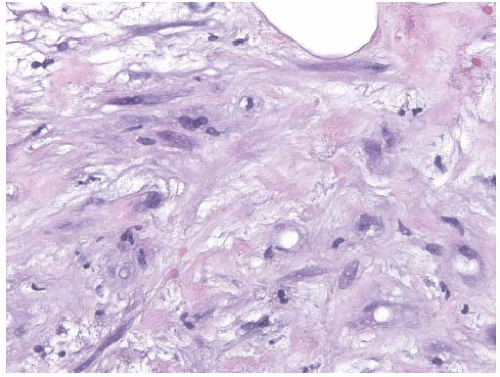
Radiation-induced histologic changes in benign breast tissue must be distinguished from recurrent carcinoma in the interpretation of a post-treatment biopsy specimen. When compared to a pre-radiation specimen, the major changes in normal breast are apparent in terminal duct-lobular units50,62,63 (Figs. 41.2 and 41.3). These include collagenization of intralobular stroma; thickening of periacinar and periductular basement membranes; severe atrophy of acinar and ductular epithelium; cytologic atypia of residual epithelial cells; and relatively prominent acinar myoepithelial cells, which seem to be preserved to a greater extent than the epithelial cells. In a minority of specimens, one may also find atypical fibroblasts in the interlobular stroma (Fig. 41.4). Apocrine epithelium is susceptible to developing severe cytologic atypia after therapeutic radiotherapy, especially in
hyperplastic foci. When evaluating a post-treatment biopsy sample, it is useful to examine the pre-treatment specimen for evidence of apocrine metaplasia. Knowledge that apocrine metaplasia was present in the pre-treatment specimen can be helpful in correctly interpreting the post-treatment biopsy with radiation atypia in apocrine metaplasia.
hyperplastic foci. When evaluating a post-treatment biopsy sample, it is useful to examine the pre-treatment specimen for evidence of apocrine metaplasia. Knowledge that apocrine metaplasia was present in the pre-treatment specimen can be helpful in correctly interpreting the post-treatment biopsy with radiation atypia in apocrine metaplasia.
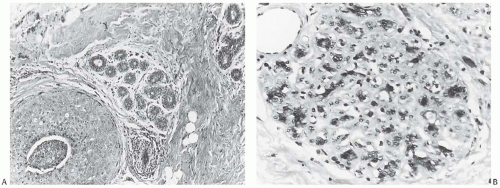 FIG. 41.3. Radiation effect in normal breast. A: DCIS and adjacent normal lobules in a 37-yearold woman. B: A lobule from the same patient after external beam radiotherapy exhibits epithelial atrophy and intralobular sclerosis. Persisting epithelial nuclei are hyperchromatic and pleomorphic. |
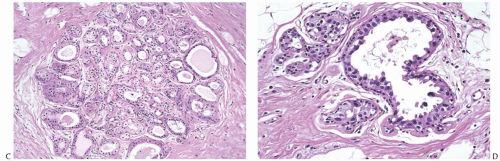
Generally, the effects on the larger ducts appear less pronounced than those in lobules (Fig. 41.5). Substantial variation in the severity of changes in the lobules can be observed from one patient to another, and on occasion the changes may be so slight as to be virtually indistinguishable from physiologic atrophy. In any one patient, most of the glandular tissue responds in a uniform fashion if the entire breast has been irradiated. Extreme variation in different parts of the breast is not commonplace. In one study, differences in radiation effects between individual patients were not correlated with radiation dose, patient age, post-treatment interval, or the use of adjuvant chemotherapy.62
Moore et al.63 studied 120 breast specimens obtained at various intervals (less than 1 year to more than 6 years) after radiotherapy. They observed statistically significant differences between pre- and post-treatment specimens, with the latter showing pathologic alterations resulting from irradiation. However, radiation-induced changes did not vary significantly in the time intervals, indicating absence of regression.
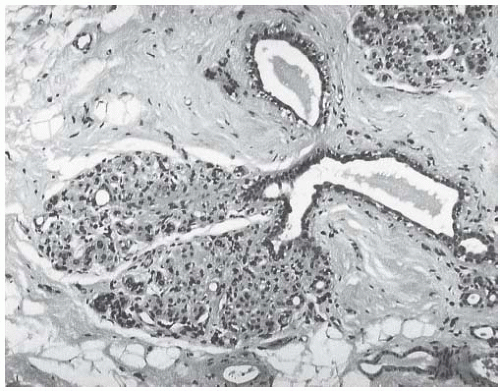 FIG. 41.5. Duct and lobule in irradiated breast. In contrast to lobular sclerosis, the duct in this post-irradiation breast appears unaffected |
When studied in vitro, atypical fibroblasts isolated from irradiated human mammary stroma expressed oncofetal fibronectin and an α-actin isoform specific for smooth muscle cells indicative of myofibroblastic differentiation.64 A study of benign breast tissue samples obtained before and
after adjuvant radiotherapy for breast carcinoma revealed increased immunohistochemical expression of p53 and increased proliferative activity assessed by Ki67 expression as long as 10 years after radiotherapy.65
after adjuvant radiotherapy for breast carcinoma revealed increased immunohistochemical expression of p53 and increased proliferative activity assessed by Ki67 expression as long as 10 years after radiotherapy.65
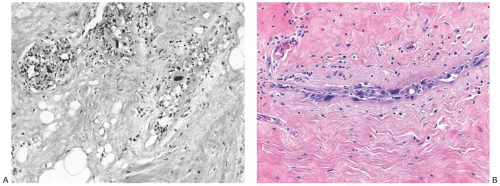 FIG. 41.6. Radiation atypia in ducts. A: Epithelial atrophy is evident. Note large atypical cells. B: Luminal cells lining the ducts have pleomorphic, large, irregular nuclei. |
When a radioactive implant or external boost has been used to give more intense treatment to the biopsy site, histologic changes in this area may be more severe than those in the surrounding breast. Fat necrosis and atypia of stromal fibroblasts are more common in proximity to such “boosted” or implanted areas.50,55,56 Radiation-induced vascular changes are not ordinarily seen after external beam radiotherapy, but they may occur when a boost dose has been delivered. Cytologic and architectural indications of radiation effect in larger blood vessels include fragmentation of elastica, endothelial atypia, and myointimal proliferation that leads to vascular sclerosis. Prominent, cytologically atypical endothelial cells are also apparent in capillaries. In boosted areas, epithelial atypia may occur in the larger ducts of the breast and it may even be superimposed on existing hyperplasia (Fig. 41.6) or apocrine metaplasia (Fig. 41.7).
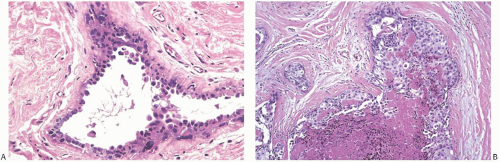 FIG. 41.7. Radiation atypia in duct with apocrine metaplasia. A: Epithelial nuclei are enlarged and hyperchromatic. Images (B-D) are from the same patient. B: In 1994, the patient underwent breast-conserving surgery and radiotherapy for DCIS shown here. C: Coincidental with DCIS in 1994, the breast also had foci of apocrine metaplasia shown here in a lobule. D: Biopsy performed in 1998 showed apocrine metaplasia with cytologic atypia. There was no recurrent carcinoma. |
Cytologic atypia can create diagnostic problems, even if one is aware of the typical appearance of radiation-induced atrophy of the breast.66,67,68 False-positive fine-needle aspiration (FNA) cytology diagnoses have been attributed to radiation atypia.69 In one series, the diagnostic yield with incisional or needle core biopsy was considerably higher than with aspiration cytology.70 The aspirate from a breast with radiation changes alone tends to be sparsely cellular because of treatment-induced atrophy. Filomena et al.71 reported that all carcinomas recurrent in irradiated breasts that were diagnosed by FNA had at least 5 epithelial cell clusters and more than 15 single epithelial cells
on the slides. Usually, there were two diagnostic slides per case. Cytologic features of benign irradiated epithelial cells were frequently very atypical and usually displayed nuclear enlargement, increase in the nuclear-to-cytoplasmic ratio, and prominence of the nucleoli. Loss of cohesion, irregularity of the nuclear borders, and necrosis are features associated with carcinoma.72 Although core biopsy has replaced FNA biopsy in many settings,73 the difficulties distinguishing the cytologic alterations produced by irradiation from the cellular atypia of irradiated carcinoma and tumor recurrence are also encountered in these samples.
on the slides. Usually, there were two diagnostic slides per case. Cytologic features of benign irradiated epithelial cells were frequently very atypical and usually displayed nuclear enlargement, increase in the nuclear-to-cytoplasmic ratio, and prominence of the nucleoli. Loss of cohesion, irregularity of the nuclear borders, and necrosis are features associated with carcinoma.72 Although core biopsy has replaced FNA biopsy in many settings,73 the difficulties distinguishing the cytologic alterations produced by irradiation from the cellular atypia of irradiated carcinoma and tumor recurrence are also encountered in these samples.
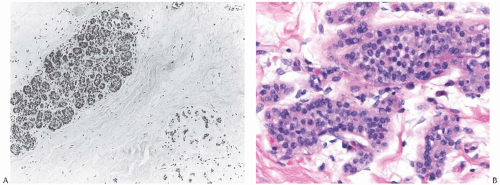
In situ lobular and ductal carcinomas persisting after radiation therapy are largely intact; consequently, the affected lobules and ducts appear filled and, often, expanded with the neoplastic population. In one study,74 the grade of recurrent DCIS was the same as the pre-treatment lesion in 95 (84%) of 113 cases. Frequently, little or no microscopic change attributable to treatment is evident when pre- and postradiation samples of in situ carcinoma are compared (Figs. 41.8 and 41.9). Greater cytologic atypia after treatment is encountered in a minority of cases. Comparison with the histologic appearance of the tumor and noncancerous tissue prior to treatment is necessary in difficult cases.
In a study of invasive breast recurrences after conservation therapy with radiotherapy in women 40 years or younger at diagnosis, Sigal-Zafrani et al.75 found no significant differences in histologic type, grade, and hormone receptor expression between primary and recurrent tumors. New carcinomas that arose outside the index quadrant were more likely to differ from the initial primary tumor than
were recurrences in the same quadrant. From time to time, irradiated invasive carcinoma cells contain multiple hyperchromatic nuclei, or there is focal necrosis that was not seen in the pre-treatment biopsy. These findings suggest that the cells represent residual carcinoma showing radiation effects. The relationship of breast irradiation to the development of angiosarcoma is discussed in Chapter 39.
were recurrences in the same quadrant. From time to time, irradiated invasive carcinoma cells contain multiple hyperchromatic nuclei, or there is focal necrosis that was not seen in the pre-treatment biopsy. These findings suggest that the cells represent residual carcinoma showing radiation effects. The relationship of breast irradiation to the development of angiosarcoma is discussed in Chapter 39.
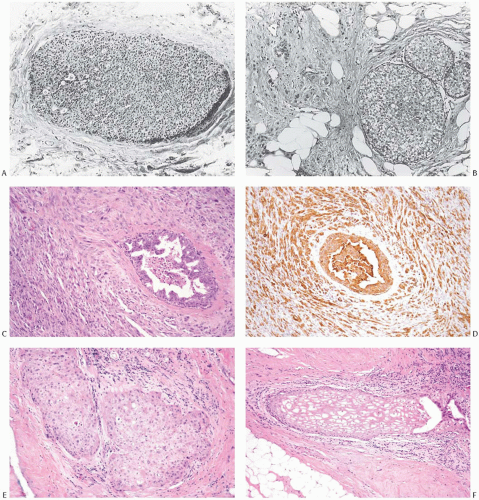 FIG. 41.9. Radiation effect in DCIS. A: DCIS prior to radiotherapy. B: Recurrent carcinoma in the same patient 2 years after treatment. The DCIS closely resembles the pre-treatment lesion. Invasive carcinoma is present on the left. C-F:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|