Pathologic Effects of Therapy
RADIATION
The breasts may be exposed to radiation during diagnostic procedures, such as mammography and fluoroscopy (1,2) or in the course of radiotherapy administered to another organ, such as mediastinal radiotherapy for Hodgkin’s disease (3,4,5,6). The low-dose exposure in these situations has been associated with an increased risk for the subsequent development of breast carcinoma (1,2,3). Wendland et al. (7) reported that the standard incidence ratio (SIR) for breast carcinoma among Hodgkin’s disease patients who received radiotherapy was 3.17, with a 95% confidence interval (CI) of 2.66-3.79 when compared to the general population. The SIR for radiated female Hodgkin’s disease patients compared to nonradiated patients was 1.90. In the same study, the SIR for breast carcinoma in nonradiated Hodgkin’s disease patients, when compared to the general population, was also elevated (1.67, 95% CI:1.24-2.20). Each of these differences was statistically significant, indicating that women who had been treated for Hodgkin’s disease had an elevated risk for breast carcinoma, which was enhanced in radiated women.
No structural changes attributable to this level of radiation are evident when the mammary glandular tissue is examined histopathologically. Patients treated for Hodgkin’s disease have carcinomas that are not significantly different pathologically from tumors that arise in women without prior irradiation (5), but the carcinomas are more likely to be bilateral and to occur in the medial part of the breast (8,9).
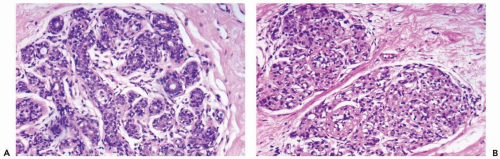
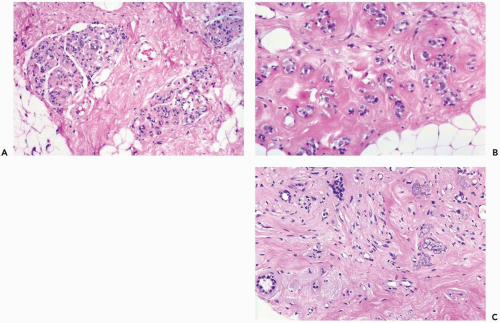
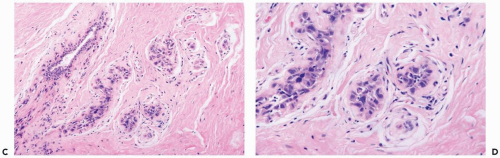
Radiation of the breast for mammary carcinoma in the course of breast conserving treatment involves levels of exposure that produce alterations in nonneoplastic as well as neoplastic breast tissues. Radiation-induced histologic changes must be distinguished from recurrent carcinoma in the interpretation of a posttreatment biopsy specimen. When the normal breast is compared to a preradiation specimen, the major changes in normal breast are apparent in terminal duct-lobular units (10) (Figs. 26-1, 26-2 and 26-3). These are (a) collagenization of intralobular stroma, (b) thickening of periacinar and periductular basement membranes, (c) atrophy of acinar and ductular epithelium, (d) cytologic atypia of residual epithelial cells; and (e) relatively prominent acinar myoepithelial cells that tend to be preserved to a greater extent than the epithelial cells. Generally, the effects on the larger ducts are less pronounced than are those in lobules following primary radiotherapy. Apocrine epithelium is susceptible to developing severe cytologic atypia after therapeutic radiotherapy, especially in hyperplastic foci. When evaluating a posttreatment biopsy, it is useful to examine the pretreatment specimen for evidence of apocrine metaplasia. In a minority of specimens, one may also find atypical fibroblasts in the interlobular stroma.
Substantial variation in the severity of changes in the lobules can be observed from one patient to another, and, on occasion, they may be virtually indistinguishable from physiologic atrophy. In a given patient most of the glandular tissue responds in a uniform fashion if the entire breast has been radiated.
Moore et al. (11) studied 120 breast specimens obtained at various intervals (less than 1 year to more than 6 years) after radiotherapy. They observed statistically significant differences between pretreatment and posttreatment specimens, with the latter showing the aforementioned alterations resulting from radiation. However, radiation-induced changes did not “show significant alterations over the various time intervals” indicating absence of regression.
Fat necrosis and atypia of stromal fibroblasts are more common close to “boosted” or implanted areas (12). Radiation-induced vascular changes are not ordinarily seen after external beam radiotherapy, but they may occur where a boost dose has been delivered. Cytologic and architectural markers of radiation effect in larger blood vessels include fragmentation of elastica, endothelial atypia, and myointimal proliferation that leads to vascular sclerosis.
Prominent, cytologically atypical endothelial cells are also apparent in capillaries. Epithelial atypia may occur in the larger ducts of the breast where it is usually superimposed on preexisting hyperplasia or apocrine metaplasia (Figs. 26.4, 26.5 and 26.6).
Prominent, cytologically atypical endothelial cells are also apparent in capillaries. Epithelial atypia may occur in the larger ducts of the breast where it is usually superimposed on preexisting hyperplasia or apocrine metaplasia (Figs. 26.4, 26.5 and 26.6).
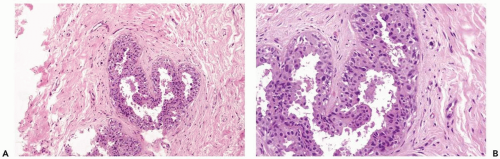
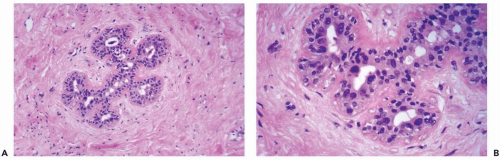 Figure 26.3 Radiation atypia in a small duct. A, B. Isolated epithelial cells bordering on the duct lumen have enlarged hyperchromatic nuclei. Thickening of the basement membrane is evident. |
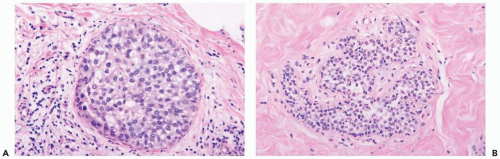
Cytologic atypia in nonneoplastic epithelium can create diagnostic problems if one is unaware of the typical appearance of radiation-induced atrophy of the breast (10,13,14). In situ lobular and intraductal carcinoma persisting after radiation therapy remain largely intact so that the affected lobules and ducts are filled and often expanded with a neoplastic cell population that differs little, or not at all, from the pretreatment appearance of the carcinoma (Figs. 26.7




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree