Anatomy and Physiologic Morphology
EMBRYOLOGY AND INFANTILE BREAST DEVELOPMENT
The mammary glands develop from the mammary ridges or milk lines, thickenings of the epidermis that appear on the ventral surface of the 5-week fetus, extending from the axilla to the upper medial region of the thigh. In the human, most of the ridge does not grow further and disappears during fetal development. Persistence of segments of the milk line is the embryologic anlage for the development of ectopic mammary glandular tissue, which occurs most often at the extreme ends of the mammary ridge in the axilla or vulva. The development of the fetal breast is characterized by the differential expression of keratins 14, 18, and 19 and of actin in the breast ducts and lobular buds. Myoepithelial cells appear to arise from basal cells between weeks 23 and 28 of gestation (1).
In most girls, functional breast development does not begin until puberty. Premature thelarche is the unilateral or bilateral appearance of a discoid subareolar thickening prior to puberty. The incidence in white female infants and children up to age 7 years in the United States in 1980 was 20.8 per 100,000 (2). The nodular breast tissue formed in premature thelarche measuring 1.0 to 6.5 cm tends to regress slowly over the subsequent 6 months to 6 years (2). Excision of this tissue is inappropriate because of the resultant amastia after puberty. Histologically, the breast tissue in premature thelarche resembles gynecomastia because it is characterized by epithelial hyperplasia in the duct system with a solid and micropapillary configuration. Growth and branching of the proliferating ducts result in an increased number of duct cross sections surrounded by moderately cellular stroma.
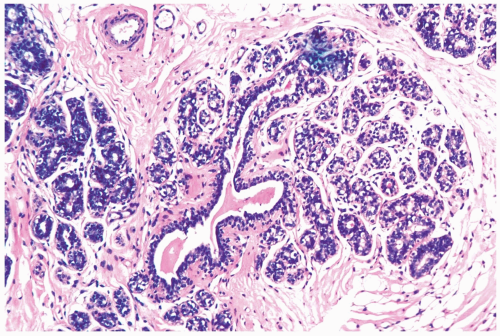
With the onset of cyclical estrogen and progesterone secretion at puberty, adolescent female breast development commences (Fig. 1.1). The growth of ducts and periductal stroma is estrogen-dependent (3). Lobules are derived from solid masses of cells that form at the ends of terminal ducts. Whereas the greatest amount of breast glandular differentiation occurs during puberty, the process may continue into the 20s and is enhanced by pregnancy.
ANATOMY AND HISTOLOGY
The functional glandular and ductal elements are embedded in fibrofatty tissue that forms the bulk of the mammary gland. The relative proportions of fat and collagenous stroma vary greatly among individuals and with age. The combination of stromal and epithelial components is responsible for the radiographic appearance of breast structure in normal and pathologic states. Magnetic resonance (MR) imaging provides a relatively precise method for discriminating between fatty and fibroglandular tissue in the breast. By comparing images obtained with mammography and MR, Lee et al. (4) found a mean fat content of 42.5% (SD ±30.3%) in mammograms and 66.5% (SD ±18%) in MR images. The ranges of fat content obtained by mammography and MR imaging were 7.5% to 90% and 17% to 89%, respectively. The correlation coefficient for estimates of fat content obtained by both methods was 0.63, with the strongest correlation (r = 0.81) in postmenopausal women.
Each of the major lactiferous ducts terminates in and exits from the breast at the nipple via a secretory pore forming the lactiferous duct orifice. The squamocolumnar junction, where the squamous epithelium joins the glandular duct epithelium, is normally distal to a dilated segment of the lactiferous duct (lactiferous sinus) located just beneath
the nipple surface. Extension of squamous epithelium into or below the lactiferous sinus is a pathologic condition termed squamous metaplasia. This may result in obstruction of the affected duct system.
the nipple surface. Extension of squamous epithelium into or below the lactiferous sinus is a pathologic condition termed squamous metaplasia. This may result in obstruction of the affected duct system.
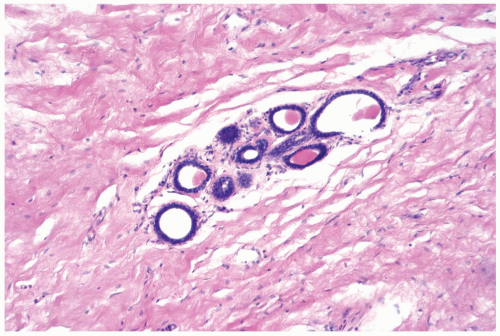 Figure 1.1 Immature breast. Breast tissue at the onset of puberty in an 11-year-old girl showing early lobular differentiation with glandular secretion and intralobular stroma. |
Lactiferous ducts in the nipple are surrounded by circular and longitudinal arrays of smooth muscle fibers embedded in fibrocollagenous stroma. The lactiferous ducts extend distally from the nipple through a series of branches diminishing in caliber from the nipple to the terminal ductal-lobular units, which are embedded in specialized, hormonally responsive stroma. Extralobular ducts are lined by columnar epithelium that is supported by myoepithelial cells, a basement membrane, and surrounding elastic fibers. In the nonlactating breast, the major ducts cut in cross section have contours marked by numerous folds or indentations that create a foliate or serrate border. The epithelium in the bay-like extensions of the duct lumen may give rise to ductular branches. Fully formed lobules can originate directly from this anatomic arrangement in the nipple and at deeper levels of the mammary duct system (5).
The majority of the cells that form the duct epithelium are columnar cells lining the lumen. Their cytoplasm is endowed with abundant organelles involved in secretion. Myoepithelial cells lie between the epithelial layer and the basal lamina. The cytoplasm of the myoepithelial cells, distributed in a network of slender processes that invest the overlying epithelial cells, is rich in myofibrils. The histologic appearance and immunoreactivity of myoepithelial cells are variable, especially in pathologic conditions, and depends on the degree to which the myoid or epithelial phenotype is accentuated in a particular situation. Regardless of their phenotypic appearance, myoepithelial cells display nuclear reactivity for p63, which is the most useful marker for detecting these cells in normal and lesional tissue.
The normal periductal stroma contains fibroblasts, elastic fibers, a sparse scattering of lymphocytes, plasma cells, mast cells, and histiocytes. Ochrocytes are periductal histiocytes with a cytoplasmic accumulation of lipofuscin pigment. These pigmented cells become more numerous in the postmenopausal breast and in association with inflammatory or proliferative conditions (6).
Mammary secretion originates in the lobules. These structures are composed of groups of alveolar glands encompassed by specialized vascularized stroma. The alveoli are connected by intralobular ductules that combine to form a single, terminal lobular duct that drains into the extralobular duct system. The resting lobular gland is lined by a single layer of cuboidal epithelial cells supported by loosely connected myoepithelial cells. The normal microscopic anatomy of the lobules is not constant because the structure and histologic appearance of the lobule in the mature breast is subject to changes associated with the menstrual cycle, pregnancy, lactation, exogenous hormone administration, and the menopause. Furthermore, there is variation in the functional state of individual lobules regardless of physiologic circumstances, an observation that suggests that individual lobules or regions of the breast have intrinsic differences in response to hormonal stimuli. This is reflected in the substantial variability in labeling indices, indicating different proliferative rates among lobules in a given individual (7). Immunoreactivity for hormone receptors is also variably expressed in lobules.
PHYSIOLOGIC MORPHOLOGY
Histologic cellular and structural alterations occur in the normal breast during the menstrual cycle (8). According to some authors, the proliferative phase, days 3 through 7, features the highest rate of epithelial mitosis and of apoptosis (9,10). Other investigators who defined this phase as days 0 to 5 reported that “apoptosis and mitosis were by and large absent in this phase” (8). Lobular glands, at this time, are lined by crowded, poorly oriented epithelial cells with little or no lumen formation and secretion. Myoepithelial cells are inconspicuous and difficult to distinguish from epithelial cells. The lobular stroma is relatively dense and hypovascular, with plump fibroblasts ringing lobular glands.
Mitoses and apoptic bodies are inconspicuous in the follicular phase, days 8 through 14. At this stage, the myoepithelial cells have a polygonal shape, clear cytoplasm, and become more apparent. Epithelial cells become columnar, with increasingly basophilic cytoplasm and basally oriented, darkly stained nuclei. An acinar lumen without secretion is evident.
During the luteal phase, comprising days 15 through 20, myoepithelial cells become more prominent due to increased glycogen accumulation that results in cytoplasmic
clearing. The glandular lumen is clearly defined by epithelial cells with basophilic cytoplasm. A small amount of secretion is present in a few glands. Edema and a mixed inflammatory cell infiltrate appear in the intralobular stroma. Mitoses and apoptotic bodies are infrequent.
clearing. The glandular lumen is clearly defined by epithelial cells with basophilic cytoplasm. A small amount of secretion is present in a few glands. Edema and a mixed inflammatory cell infiltrate appear in the intralobular stroma. Mitoses and apoptotic bodies are infrequent.
The secretory phase corresponding to days 21 through 27 features heightened secretion with distension of glandular lumens by accumulated secretory material. The epithelium consists of epithelial cells and myoepithelial cells with progressively clear cytoplasm caused by the accumulation cytoplasmic vacuoles (Fig. 1.2). At this stage, mitoses and apoptotic bodies are most conspicuous, with maximal intralobular edema and inflammation.
In the menstrual phase, the stroma becomes compact with loss of intralobular edema. Lymphocytes, macrophages, and plasma cells are most conspicuous in the lobular stroma at this stage (9). Some glandular lumens remain and others appear collapsed. Mitotic activity is absent.
Improved ability to recognize morphologic changes in the breast that are related to the menstrual cycle may become clinically useful in premenopausal women. At present, evidence suggesting that surgery performed during the luteal phase is prognostically advantageous (11,12




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree