Tubular Carcinoma
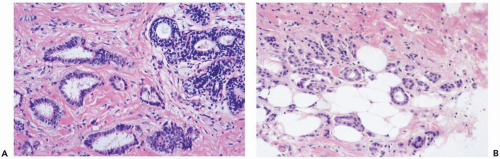
Tubular carcinoma is “a highly differentiated invasive carcinoma whose cells are regular and arranged in well-defined tubules typically one cell-layer thick and surrounded by an abundant fibrous stroma” (1) (Fig. 10.1). The term tubular refers to the formation of neoplastic glands that resemble breast ductules. This characteristic is the main source of difficulty in recognizing the lesion, especially in the limited sample of a needle core biopsy (Fig. 10.2).
The development of laser microdissection has made it possible to obtain relatively pure microscopic samples of neoplastic glandular tissue for molecular analysis. In a study of 18 pure tubular carcinomas, sampled by laser microdissection and investigated by comparative genomic hybridization, Waldman et al. (2) detected an average of 3.6 chromosomal alterations per tumor. Tubular carcinomas had significantly fewer chromosomal alterations overall and significantly more frequent 16q gains when compared to a group of nontubular invasive duct carcinomas. The authors concluded that pure tubular carcinomas are a distinct form of mammary carcinoma that may not evolve into less well-differentiated tumors.
The diagnosis of tubular carcinoma does not apply to carcinomas in which tubular structures are formed by less well-differentiated cells or have a more complex growth pattern (Fig. 10.3). The pattern of glandular growth in nontubular well-differentiated duct carcinoma is partly tubular but in areas the proliferation is more florid and the epithelium of the glands may be more than one-cell thick. Some glands develop micropapillae, cribriform transluminal bridging, or microgland structures. In pure tubular carcinoma an occasional mitotic figure may be encountered, but there is little cytologic pleomorphism. The intraductal component is usually cribriform or micropapillary in tubular carcinoma. Often the intraductal proliferation at the site of tubular carcinoma is so orderly that it is indistinguishable from atypical duct hyperplasia.
Pure tubular carcinomas constitute less than 2% of all female breast carcinomas. The incidence of this lesion is influenced by the nature of the population under investigation. Before the widespread use of mammography, 5% of 382 T1NoMo breast carcinomas in one series were classified as tubular (3). When stratified by size, 9% of lesions 1 cm or smaller were tubular, a substantially higher proportion than the 2% of tubular carcinomas among tumors that measured 1.1 to 2 cm. Tubular carcinoma was less frequent among women with T1N1Mo disease (1.5% of 142 patients), reflecting the relatively low frequency of axillary metastases from these tumors (4). In the Breast Cancer Detection Demonstration Projects, 8% of invasive carcinomas 1 cm or less in diameter were of the tubular variety (5).
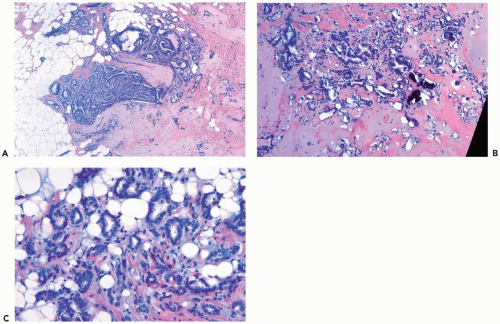
Tubular carcinomas have structural features that render the lesions detectable by mammography. Most tubular carcinomas are spiculated, and calcifications are often present (6). Leibman et al. (7) reported that the average radiographic size of tubular carcinomas was 0.8 cm in patients with nonpalpable lesions and 1.2 cm when the lesions were palpable. It may be difficult to distinguish tubular carcinoma from radial scar in mammograms, a problem compounded by the fact that tubular carcinoma can arise in a radial scar (8,9) (Fig. 10.4).
The median age at diagnosis is mid- to late 40s (10,11). Superficial tumors are fixed to the skin, producing retraction in a small number of cases. When tubular carcinoma arises from the major lactiferous ducts in the nipple or slightly lower in the subareolar region, it is difficult to distinguish from florid papillomatosis of the nipple. The finding of Paget’s disease supports the diagnosis of tubular carcinoma in this setting. Almost all tubular carcinomas have been positive for estrogen and progesterone receptors (12) (Fig. 10.5).
The distribution of the small neoplastic glands or tubules is largely haphazard, but they tend to be relatively evenly spaced throughout the lesion. An important clue to the diagnosis of tubular carcinoma that should be searched for in a needle core biopsy specimen is evidence of lesional glands invading fat beyond fibrous stroma (Fig. 10.4C). Although this distribution can occur in adenosis or a radial sclerosing lesion, it is more likely to be found in tubular carcinoma.
The glands may have virtually any shape, but the majority of the glands have irregular shapes and angular contours (Fig. 10.2). In exceptionally well-differentiated tubular carcinomas, especially tumors smaller than 1 cm, it is possible to find round or oval glands of relatively uniform caliber that resemble microglandular adenosis (13,14) (Fig. 10.2B). If cribriform and papillary growth are present in the neoplastic glands, the tumor is best classified as
invasive well-differentiated duct carcinoma rather than as pure tubular carcinoma (Fig. 10.3).
invasive well-differentiated duct carcinoma rather than as pure tubular carcinoma (Fig. 10.3).
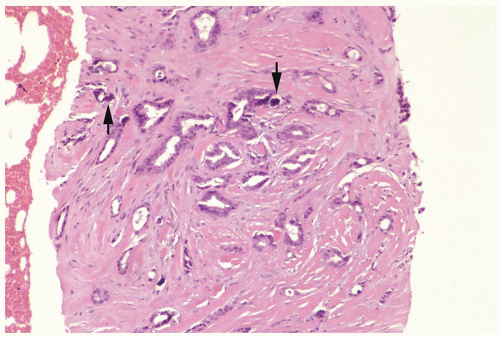 Figure 10.1 Tubular carcinoma. This needle core biopsy specimen shows small, evenly distributed glands. Calcifications are visible (arrows). |
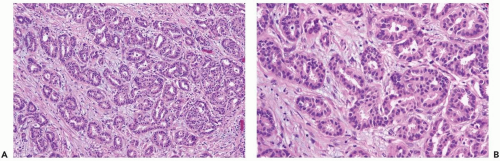 Figure 10.3 Well-differentiated invasive duct carcinoma. A, B. The glandular pattern is more complex than in tubular carcinoma. Intraglandular proliferation is evident. |
The glands of tubular carcinoma are composed of only neoplastic epithelial cells, typically distributed in a single layer (Fig. 10.6). The cells are cuboidal or columnar with round or oval hyperchromatic nuclei that tend to be basally oriented. Nucleoli are inapparent and mitoses are very rarely present. Apocrine-type cytoplasmic “snouts” are often evident at the luminal cell border. The cytoplasm is usually amphophilic. Eosinophilic or nearly clear apocrine cytoplasm rarely occurs in a pure tubular carcinoma. An uncommon type of tubular carcinoma is composed of tumor cells with intracytoplasmic mucin and mucin in the neoplastic gland lumens (Fig. 10.7).
The primary considerations in the differential diagnosis of tubular carcinoma are sclerosing adenosis and radial sclerosing




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree