Invasive Duct Carcinoma
This is the largest group of malignant mammary tumors constituting 75% to 80% of mammary carcinomas. A generic term sometimes used is invasive duct carcinoma, not otherwise specified (NOS). This is a useful designation that recognizes the distinction between these tumors and other specific forms of duct carcinoma, such as tubular, medullary, metaplastic, colloid, and adenoid cystic carcinoma.
Invasive duct carcinoma includes a subset of tumors that expresses, in part, characteristics of one of the specific types of breast carcinoma but do not constitute pure examples of the individual tumors. One example of this phenomenon is invasive duct carcinoma with lobular carcinoma features (Fig. 9.1). Foci of tubular, mucinous, or papillary differentiation can be found in invasive duct carcinomas. When evidence of a mixed growth pattern is present in a needle core biopsy, the findings should be reported descriptively, with final classification reserved for the excisional biopsy. The relatively favorable prognosis associated with some specific histologic types has been found to apply only to those tumors composed entirely or in very large part of the designated pattern. Invasive duct carcinoma with a minor portion of a specific subtype is likely to have the prognosis of the dominant invasive duct carcinoma component.
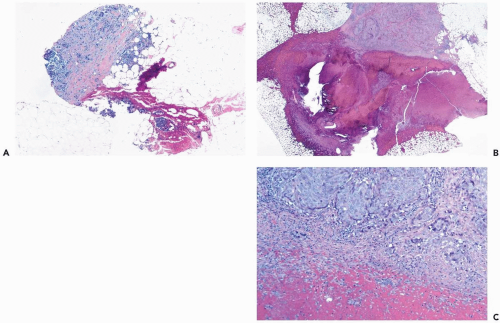
The specimens obtained in a needle core biopsy procedure often include multiple samples of tumor tissue (Fig. 9.2). On occasion, only a small fragment of the lesion may be present (Fig. 9.3). All tissue on a slide must be carefully examined to ensure that this very limited material is not overlooked. In the most extreme circumstance, the evidence is so scant that a diagnosis of carcinoma cannot be made with confidence, and an excisional biopsy is necessary to determine whether carcinoma is present.
Although it is technically possible to examine needle core biopsy specimens by frozen section, this is not a standard practice and should only rarely be done in exceptional situations when there is strong clinical evidence that carcinoma is present and there are compelling clinical circumstances that warrant immediate intervention after the diagnosis has been rendered (Fig. 9.4). Major reasons for avoiding frozen section diagnosis of needle core biopsy samples are the loss of tissue from these small specimens during slide preparation and the potential for misdiagnosis of a number of benign lesions that mimic carcinoma. The concern over interpretive issues that apply to these lesions when they are excised intact and examined by frozen section is enhanced in the disrupted form presented by needle core biopsy samples.
No specific clinical features distinguish invasive duct carcinoma from other types of invasive carcinoma and some benign tumors. The lesions occur throughout the age range of breast carcinoma. Invasive duct carcinoma typically forms a solid tumor. Cystic change in this group of lesions is extremely uncommon but may be a manifestation of necrosis.
The measured gross size of a mammary carcinoma is one of the most significant prognostic variables. Survival decreases with increasing tumor size of invasive duct carcinoma and most of the subtypes of breast carcinoma, and there is a coincidental rise in the frequency of axillary nodal metastases (1,2). This phenomenon applies not only to the overall spectrum of primary tumor size, but also within subsets, such as those defined by tumor-node-metastasis (TNM) staging. For example, among T1 breast carcinomas (less than or equal to 2 cm in diameter), there is a significant relationship between size, the frequency of nodal metastases, and prognosis when the tumors are stratified in 5-mm groups (3,4). The interaction of the number of involved lymph nodes and tumor size is important prognostically in stage II patients. Quiet et al. (5) found that the long-term disease-free survival after mastectomy was 81% in patients with one lymph node metastasis and a tumor 2 cm or smaller, compared with 59% if the tumor was larger than 2 cm.
Because many carcinomas have asymmetric shapes, the measurement of size is generally reported in terms of the greatest diameter. The gross measurement of the size of a carcinoma is only an approximation of the actual amount of invasive tumor present (6,7). Measurement of the
invasive component exclusive of peripheral extensions of intraductal carcinoma is recommended when it is practical. It is rarely possible to measure the size of an invasive carcinoma in a needle core biopsy because it is difficult to ensure that the sample represents a complete diameter in the largest dimension. When microinvasive ductal carcinoma is found in a needle core biopsy, it is possible that a larger invasive lesion will be found when an excisional biopsy is performed. A small invasive focus may be limited to one of multiple levels prepared from a paraffin block (8). Correlation of the needle core biopsy, with the dimensions of a small lesion seen on a mammogram, can be useful in some circumstances to confirm the size of a carcinoma and to assess the contour of the tumor.
invasive component exclusive of peripheral extensions of intraductal carcinoma is recommended when it is practical. It is rarely possible to measure the size of an invasive carcinoma in a needle core biopsy because it is difficult to ensure that the sample represents a complete diameter in the largest dimension. When microinvasive ductal carcinoma is found in a needle core biopsy, it is possible that a larger invasive lesion will be found when an excisional biopsy is performed. A small invasive focus may be limited to one of multiple levels prepared from a paraffin block (8). Correlation of the needle core biopsy, with the dimensions of a small lesion seen on a mammogram, can be useful in some circumstances to confirm the size of a carcinoma and to assess the contour of the tumor.
A review of tumor sizes determined in mammograms and excised specimens found no evidence that a prior
needle core biopsy influenced the measurement of the tumors (9). The study evaluated 138 mammographically detected T1 tumors, including 61 sampled by core biopsy prior to excision and 77 excised without a core biopsy. The mean differences between mammographic and pathologic sizes in the two groups were not significantly different (2.3 mm and 1.96 mm, respectively).
needle core biopsy influenced the measurement of the tumors (9). The study evaluated 138 mammographically detected T1 tumors, including 61 sampled by core biopsy prior to excision and 77 excised without a core biopsy. The mean differences between mammographic and pathologic sizes in the two groups were not significantly different (2.3 mm and 1.96 mm, respectively).
A minority of patients have a clinically or grossly identifiable multifocal tumor in the same breast quadrant, or multicentric tumors in more than one quadrant. When clinically apparent, multiple nodules may be sampled by needle core biopsies to confirm this impression. Data obtained by Andea et al. (10) suggest that the aggregate diameter of grossly measurable nodules should be used to determine tumor size or T-stage rather than the past convention of staging on the basis of the largest nodule. Calculated aggregate tumor volumes and surface areas were the best predictors of nodal status. As noted previously, needle core biopsy samples are not a reliable basis for measuring tumor diameter.
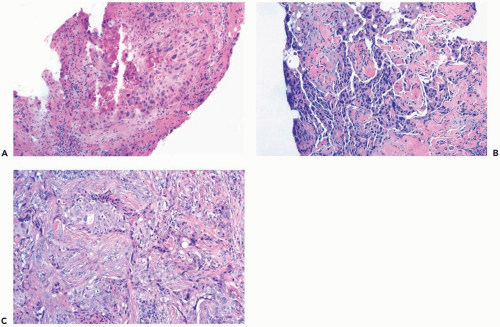
Histologic grading of invasive duct carcinomas is an estimate of structural differentiation, limited to the invasive portion of the tumor (Fig. 9.5). The most widely used histologic grading system is based on criteria established by Bloom and Richardson (11) and Elston and Ellis (12). The parameters measured are the extent of tubule formation, nuclear hyperchromasia or size, and mitotic rate. Histologic grade is usually expressed in three categories: well differentiated (grade I), intermediate (grade II), and poorly differentiated (grade III). A modified Scarff-Bloom-Richardson system presented by Robbins et al. (13) is outlined in Table 9.1.
Nuclear grading is the cytologic evaluation of tumor nuclei in comparison with the nuclei of normal mammary epithelial cells (Fig. 9.6). Because nuclear grading does not involve an assessment of the growth pattern of the tumor, this procedure is applicable not only to invasive duct carcinoma, but also to other subtypes of mammary carcinoma. The most widely used system for nuclear grading, introduced by Black and Speer (14) and Cutler et al. (15), is usually reported in terms of three categories: well differentiated, intermediate, and poorly differentiated. By convention, the sequence of numeric designations originally used for nuclear grading was the reverse of histologic grading, but, more recently, there has been a trend to use a numbering system that conforms with histologic grading (grade 1, well differentiated; grade 2, moderately differentiated; grade 3, poorly differentiated). Because of the potential for confusion on this issue, it is preferable to use descriptive terms for nuclear grading rather than numerals.
Several studies have investigated the accuracy of histologic grading based on needle core biopsy specimens when compared to the final grade determined from the excised tumor. The reported concordance rates ranged from 59% to 75% (16,17,18). In the same studies, concordance with respect to tumor type ranged from 66.6% to 81.0%. These data suggest that classification and grading of invasive carcinomas based on the needle core biopsy sample should be regarded as provisional. This consideration should be borne in mind when neoadjuvant therapy is administered on the basis of a diagnosis made with a needle core biopsy sample. Tumor heterogeneity is the most common source of discordant classification and grading, but interobserver and intraobserver variations are probably also factors.
The histologic and nuclear grades of a given tumor coincide in many, but not all, invasive duct carcinomas (19). Nuclear and histologic grade have been shown to be useful predictors of prognosis for patients stratified by stage of disease, especially those without axillary lymph node metastases (20,21). Increasing tumor grade has been associated with several factors that are related to an increased risk for breast recurrence after conservation therapy, including greater tumor size, diagnosis at the relatively young age, and absence of estrogen receptor expression. Although some investigators found a significant relationship between grade and local recurrence (22), others concluded that grade was not a significant predictor of local recurrence (23). In patients with relatively favorable stage I carcinomas treated by lumpectomy without radiotherapy, the tumor recurred sooner and with greater frequency after a median follow-up of 58 months in patients with high-grade carcinomas (24).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree