CHAPTER 11 Pain of Spinal Origin
The purpose of this chapter is to apply much of the information from previous chapters to the clinical setting. This is accomplished by discussing the case of a typical patient with low back pain. In addition, structural features of other regions of the spine particularly susceptible to injury or pathologic conditions also are mentioned briefly. The aspects of pain discussed in this chapter are meant to include the most common causes of discomfort. Exhaustive lists are beyond its scope. Fortunately, this subject has been reported thoroughly elsewhere (Kirkaldy-Willis, 1988a; Haldeman, 1992; Cavanaugh, 1995; Greenspan, 1995; Bogduk, 1997; Siddal and Cousins, 1997). This chapter discusses a challenging problem that faces clinicians continuously: their patients’ pain.*
PERCEPTION OF PAIN
The general approach to patients complaining of pain is that it is real. It has both physical and psychologic components, one of which may predominate, and the pain always alters the personality of the individual (Kirkaldy-Willis, 1988b; Burton et al., 1995; Gillette, 1996; Kummel, 1996; Hildebrandt et al., 1997). This alteration of personality usually returns to the prepain state when the physical cause of the discomfort has sufficiently healed. In addition, pain always has a subjective component and is perceived by patients in relation to previous experiences with pain, usually from their early years (Weinstein, 1988).
Pain has been defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (Merskey, 1979). This group’s committee on taxonomy goes on to state, “If a patient regards their experience as pain and if they report it in the same ways as pain caused by tissue damage, it should be accepted as pain” (Merskey, 1979). Therefore not all pain is the result of a nociceptive stimulus received and transmitted by a sensory receptor of a peripheral nerve (Weinstein, 1988).
Many other factors may influence the patient’s perception of pain, including the following: the individual’s general health, the nervous system’s overall status, the pain’s chronicity, and even the environment in which the patient lives (Haldeman, 1992). In addition, a person’s work environment, work activities, and work satisfaction have all been found to affect the occurrence and outcome of back pain (Macfarlane et al., 1997; Pappageotgiou et al., 1997). Finally, the dorsal root ganglia, spinal cord, and higher centers are all capable of adjusting and regulating (modulating) painful stimuli. Therefore to continue with the example of Mr. S., the clinician may not fully appreciate and understand the severity of Mr. S.’s pain until he or she has had an opportunity to observe him on several different occasions (Kirkaldy-Willis, 1988b).
The characteristics and quality of pain, such as that experienced by Mr. S., can be important. For example, diffuse burning pain, which may or may not radiate into the lower extremity, can be of sympathetic origin. The afferent fibers of the recurrent meningeal nerve travel with sympathetic fibers. The peripheral (sensory) receptors of the nerves may be stimulated by arachnoiditis and postoperative fibrosis and could be a source of diffuse burning pain of sympathetic origin (Kirkaldy-Willis, 1988b). However, aching usually is the result of muscle tightness or soreness and frequently is relieved by stretching and short periods of rest. Other generalized lower extremity pain, excluding aching pain, often is associated with a vascular or neurogenic cause (Weinstein, 1988).
PAIN OF SOMATIC ORIGIN
The clinician now begins to consider the possible causes of Mr. S.’s current discomfort. Low back pain (LBP) can be defined as pain in the lumbar or sacral region that is “not more than a handbreadth” away from either side of the patient’s vertebral column (Bogduk, 1992). Even though LBP is one of the most common complaints seen by physicians, it is one of the most difficult to understand (Weinstein, 1988). Recall that an anatomic structure must be supplied by nociceptive nerve endings (nerve endings sensitive to tissue damage; see Chapter 9) to be a cause of LBP, and Mr. S.’s perception of his LBP greatly depends on the factors described previously. Noncutaneous nociceptors are found in muscles, tendons, joint capsules, periosteum, perivenous tissues (vasa nervorum), and several visceral tissues (Greenspan, 1997). Therefore these are all potential sources of Mr. S.’s discomfort. Also recall that nociceptors may be stimulated by mechanical, thermal, or chemical means. Because the structures that receive nociceptive innervation are able to “generate pain,” they are sometimes called pain generators. (Notice that the presence of the nociceptive endings in the damaged tissue is what actually allows the structure to function as a pain generator.) The nociceptive nerve endings respond to tissue damaging or potentially tissue damaging stimuli; that is, nociceptive nerve endings can fire before tissue damage actually occurs, thereby possibly preventing injury (Greenspan, 1997).
Frequently nociception of spinal origin is the result of damage to several structures, and the effects of hyperalgesia allow for nociception to be felt from tissues that, if injured to the same degree independently, might have gone unnoticed (Haldeman, 1992). (Hyperalgesia and other related concepts are discussed in further detail in the section Important Terms and General Concepts Related to Pain.)
Most pain has a physical cause, even though not all the structures supplied by nociceptors, and therefore capable of producing pain, are known (Haldeman, 1992). Those tissues that are supplied by nociceptive nerve endings usually can undergo a number of different pathologic processes that can lead to direct stimulation or sensitization of nociceptors (Haldeman, 1992).
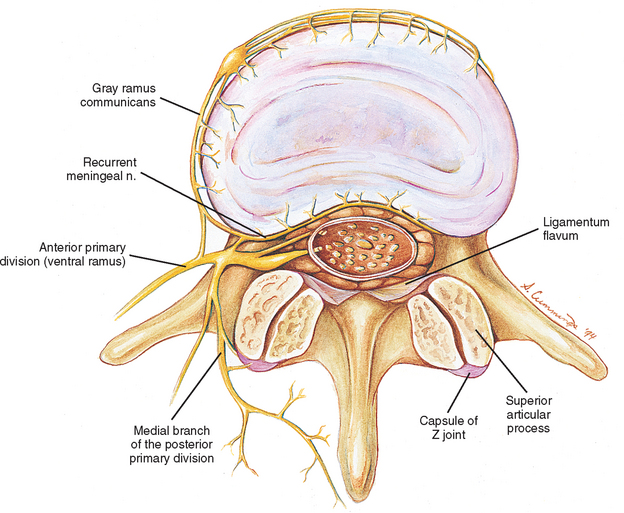
One of the best ways to organize Mr. S.’s possible pain generators is to list them according to the four main sources of neural innervation to spinal structures: the anterior primary division (APD, ventral ramus), posterior primary division (PPD, dorsal ramus), recurrent meningeal nerve, and sensory fibers that course with the sympathetic nervous system (Fig. 11-1). All these afferent nerves have their cell bodies in the dorsal root ganglia (DRGs), which, with the exception of C1 and C2 (see Chapter 5), are located within the intervertebral foramina (IVFs) of the vertebral column. Note that the sensory fibers that are associated with the recurrent meningeal nerve and sympathetic nervous system provide a route for the transmission of nociception from somatic structures of the vertebral column’s anterior aspect. Fibers arising from these sources pass through the APD for a short distance before reaching the spinal nerve. They then enter the dorsal root. Even though these nerves briefly pass through the ventral ramus, they are best considered separately because they are important to nociception of spinal origin.
Anterior Primary Divisions (Ventral Rami)
To approach the cause of the discomfort experienced by Mr. S., first consider those structures innervated by the lumbar APDs (Box 11-1). The APDs of the lumbar region innervate much of the gluteal and inguinal regions, as well as the entire lower extremity. Although these regions may refer to the low back, they usually are accompanied by more localized pain from the structure that is either injured or affected by some form of pathologic process. More likely causes of back pain originating from structures innervated by APDs (ventral rami) are several muscles, including the psoas major, quadratus lumborum, and lateral intertransversarii. Strain or possibly increased tightness (what some would call “spasm”) of these muscles can be a source of back pain. Abscess within the psoas muscle also is a possible source of pain. The transverse processes (TPs) are innervated by the APD too, and a fracture of a TP or bruise to its periosteum may result in pain (Bogduk, 1983).
Posterior Primary Divisions (Dorsal Rami)
Discomfort such as that experienced by Mr. S. also may arise from structures innervated by the PPDs (dorsal rami) listed in Box 11-2. This list contains some of the most frequent causes of LBP, including the deep back muscles, which receive nociceptive innervation by means of nerves accompanying the vessels that supply these muscles; spinal ligaments (nociceptors are most numerous in the posterior longitudinal ligament, innervated by the recurrent meningeal nerve, and fewest in the interspinous ligament and ligamentum flavum); and the zygapophysial joints (Z joints). These are all high on the list of possible causes of pain similar to that experienced by Mr. S. (Cavanaugh, 1995). Each of these groups of structures may be affected by a number of pathologic conditions or injuries. The muscles may be strained or affected by areas of myofascial tenderness (“trigger points”) (Bogduk, 1983; Hubbard and Berkoff, 1993). The ligaments may be sprained. Pain from the Z joints may be difficult to localize because each Z joint receives innervation from the PPD of the same level and also from the PPD of the level above (Bogduk, 1976) and below (Jeffries, 1988). The Z joints may be fractured (fracture of an articular process) or inflamed as a result of arthritic changes. Discomfort also can arise from a Z joint articular capsule or synovial fold (Giles, 1987; Schwarzer et al., 1994) that has become entrapped within the Z joint or pinched between the articular surfaces (see Chapter 7). Degeneration of articular cartilage may produce inflammatory agents that may stimulate nociceptors of the Z joint articular capsules. Inactivity of the spinal joints, even if this inactivity is imposed on these joints by muscle guarding, may promote degeneration (Cramer et al., 2004) (Fig. 11-2) and pain (Kirkaldy-Willis, 1988b). In addition, the spinous processes may be fractured or may repeatedly collide with one another (Baastrup’s syndrome). Finally, Sihvonen et al. (1995) found that the medial branch of the posterior primary division can become irritated or entrapped along its course, causing weakness of transversospinalis muscles (i.e., semispinalis, multifidus, and rotatores muscles) and low back pain. The LBP in this instance presumably results from irritation of the medial branch of the posterior primary division and also from abnormal stresses and loads being placed on pain generators of the spine as a result of the biomechanical changes caused by the muscle weakness.
BOX 11-2 STRUCTURES INNERVATED BY THE DORSAL RAMUS
Recurrent Meningeal Nerve
Structures innervated by the recurrent meningeal (sinuvertebral) nerve also may be a source of back pain (Raoul et al., 2002). The list in Box 11-3 identifies the structures supplied by these nerves. The periosteum of a vertebral body may be affected by fracture or neoplasm within the vertebral body. The basivertebral veins may be affected by intraosseous hypertension, crush fractures, or neoplasms of the vertebral body (Bogduk, 1983). The epidural veins may be affected by venous engorgement. The posterior aspect of the intervertebral disc (IVD) is also a pain generator (Schwarzer et al., 1994; Cavanaugh, 1995) that can be affected by internal disc disruption, protrusion of the nucleus pulposus through the outer layers of the anulus fibrosus, or tearing (sprain) of the outer layers of the anulus fibrosus (AF). Of related importance is that sensory innervation of degenerated IVDs extends into the deeper layers of the anulus fibrosus. That is, the process of IVD degeneration seems to stimulate the nerves innervating the posterior, lateral, and anterior aspects of the IVD to grow deeper into the IVD, probably making them more capable of providing nociceptive sensation (Yoshizawa, O’Brien, and Thomas-Smith, 1980). Also innervated by the recurrent meningeal nerves is the posterior longitudinal ligament, which can be torn (sprained) during severe hyperflexion injuries or may be pierced by an IVD protrusion. In addition, the anterior aspect of the dura mater may be compressed by an IVD protrusion or irritated by the release of chemical mediators associated with internal disc disruption (see Chapter 7).
Nerves Associated with the Sympathetic Nervous System
Finally, recall that several structures are innervated by nerves that arise directly from the sympathetic trunk and gray communicating rami (Box 11-4). The sensory fibers of these nerves follow the gray rami to the APD, where they enter the spinal nerve. They then reach the spinal cord by coursing through the dorsal roots. Pathologic conditions of the periosteum of the anterior and lateral aspects of the vertebral body, which are innervated by sensory fibers traveling with gray rami, may result in pain. Some of the most common causes of this type of pathologic condition include fracture, neoplasm, and osteomyelitis (Bogduk, 1983). Sprain of the anterior longitudinal ligament or outer layers of the anterior or lateral part of the anulus fibrosis also may result in nociception conducted by fibers that course with the gray communicating rami.
Under certain circumstances, the sympathetic nervous system can play a complex role with regard to pain (Jorgensen and Fossgreen, 1990; Budgell, Hotta, and Sato, 1995; Siddall and Cousins, 1997). Vascular changes, changes in perspiration (sudomotor), and changes in nail, hair, and bone structure can accompany chronic pain associated with the sympathetic nervous system (Siddall and Cousins, 1997). This pain often is burning in nature and is associated with increased stimulation of sensory receptors. This is accompanied by an increased perception of pain in areas not associated with tissue damage (hyperalgesia), and also by an increased sensitivity to mechanical stimulation, including mechanical stimulation that previously would not be considered capable of producing pain (allodynia). The mechanism may be related to the finding that nociception from damaged or regrowing nerves and even nearby axons sometimes can be activated by norepinephrine from sympathetic stimulation. This activation is thought to result from alpha-adrenergic (norepinephrine) receptors developing on C (nociceptive) fibers after partial nerve injury (Greenspan, 1997). Once known as reflex sympathetic dystrophy, the term complex regional pain syndrome is now preferred (Plancarte and Calvillo, 1997). The pain of some patients with complex regional pain syndrome is related to the sympathetic nervous system (sympathetically maintained pain), although others’ pain is not (sympathetically independent pain) (Greenspan, 1997). (See Sympathetically Maintained Pain later in this chapter and Complex Regional Pain Syndrome in Chapter 10).
Pain Generators Unique to the Cervical Region
Nociception arising from almost any structure innervated by the upper four cervical nerves may refer to the head, resulting in head pains and headaches (Campbell and Parsons, 1944; Edmeads, 1978; Bogduk, Lambert, and Duckworth, 1981; Bogduk, 1984; Aprill, Dwyer, and Bogduk, 1990; Dwyer, Aprill, and Bogduk, 1990; Darby and Cramer, 1994; Cramer, 1998). Pain originating from the region of the basiocciput and occipital condyles frequently refers to the orbital and frontal regions (Campbell and Parsons, 1944). A discussion of neck pain and its relationship to headache and head pain has been fully covered elsewhere (Vernon, 2001), and is beyond the scope of this chapter.
Autonomic reactions such as sweating, pallor, nausea, alterations of pulse, and other autonomic disturbances frequently have been observed in association with disturbances of the suboccipital and upper cervical spine. The intensity of these autonomic reactions seems to be proportional to the stimulus and proximity of the stimulus to the suboccipital region. The autonomic response ranges from mild subjective discomforts to measurable objective signs (Campbell and Parsons, 1944).
Pain Generators Unique to the Thoracic Region
If Mr. S. should present with discomfort of the thoracic region, the costocorporeal and costotransverse articulations should be added to the list of possible pain generators (see Chapter 6). Also a compression fracture of one or more of the thoracic vertebral bodies could be a realistic source of acute pain arising from the thoracic region.
Dorsal Root Ganglia
The neurons located in the dorsal root ganglia (DRG) serve as modulators of spinal nociception. They contain many neuropeptides (see Chapter 9) associated with the transmission of nociception (e.g., substance P, calcitonin gene-related peptide [CGRP], vasoactive intestinal peptide) (Weinstein, Claverie, and Gibson, 1988). These neuropeptides and other neuromodulators are secreted from the peripheral terminals of sensory nerves that transmit nociception. These substances are manufactured in the cell bodies of the DRG and reach the peripheral terminals (sensory endings) and the synaptic boutons found in the dorsal horn of the spinal cord by axonal transport mechanisms. The presence of neuropeptides and neuromodulators around the receptors may sensitize the receptors, making them more susceptible to depolarization (Weinstein, Claverie, and Gibson, 1988). (See the sections Important Terms and General Concepts Related to Pain, and Modulation of Nociception for more information on the modulation of nociception at the level of the peripheral nerve endings.)
SOMATIC REFERRED PAIN
Nociception arising from any of the somatic structures previously listed may be perceived by Mr. S. or a similar patient as being a considerable distance from the pain generator, even in an area innervated by different nerves than those innervating the pain generator. This is known as pain referral. The term somatic referred pain has been used to describe this type of back pain (Bogduk, 1992, 1997).
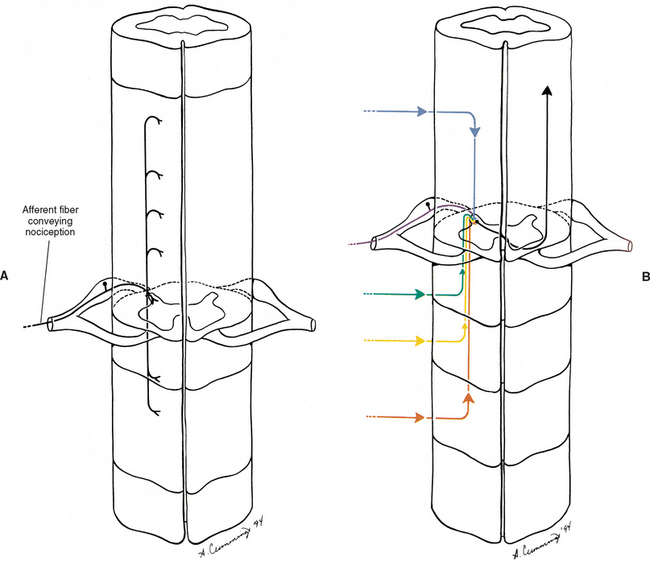
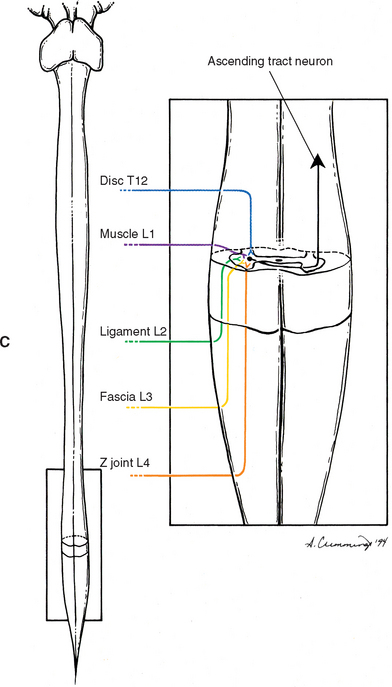
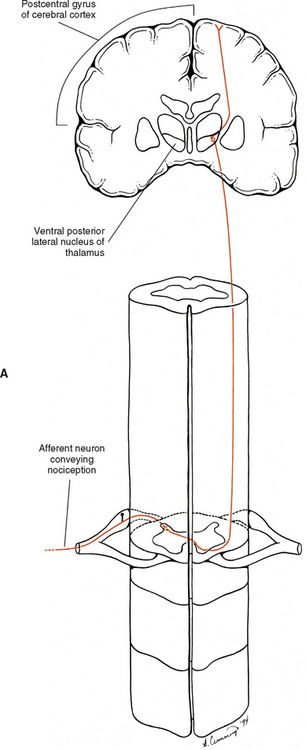
Several possible mechanisms of pain referral exist. Perhaps one of the most important mechanisms is the result of the internal organization of the spinal cord. The nociceptive information coming in from a pain generator is dispersed by either ascending or descending fibers that make up the dorsolateral tract of Lissauer (see Chapter 9). These fibers may ascend or descend several cord segments before synapsing. Thus nociceptive information, entering from several different spinal cord segments, converges on the same interneuronal pool; therefore this interneuronal pool receives primary sensory information from different somatic regions (Fig. 11-3). More specifically, the dorsal horn neurons in the extreme lateral aspect of the dorsal gray horn have been found to receive input from a wide variety of superficial and deep tissues. In fact, spinal tissues have been found to produce more convergence in the spinal cord than other tissues, a phenomenon termed hyperconvergence (Gillette, Kramis, and Roberts, 1993). Gillette, Kramis, and Roberts (1993) found that neurons within the skin, Z joint, spinal ligaments, and paraspinal muscles all caused firing of the same dorsal horn neurons. This dispersal of incoming afferents onto different-tract neurons, in combination with the convergence of several different afferents onto single tract neurons, most likely decreases the ability of the CNS to localize nociception (Haldeman, 1992; Darby and Cramer, 1994). In addition, excitatory and inhibitory interneurons found in laminae I and II in the spinal cord can be activated by nonnociceptive afferents, as well as by descending pathways, and also can modulate the output of second-order neurons, thus further altering the ability of the CNS to localize nociception. This type of dispersal and convergence also may be found at the second synapse along the nociceptive pathway. That synapse occurs in the ventral posterior lateral nucleus of the thalamus (see later discussion on pain pathway).
The ventral posterior lateral thalamic nucleus projects to the postcentral gyrus of the cerebral cortex. The region of the back is represented on a small area of the postcentral gyrus (sensory homunculus) of the cerebral cortex (see Fig. 9-5). The small size of the sensory cortex devoted to the back also may contribute to the poor localization of nociception of spinal origin (Haldeman, 1992). In addition, the tract neurons for ascending pain pathways most frequently carry nociceptive information from cutaneous areas. Therefore when the tract neurons are stimulated to fire, the cerebral cortex (where conscious awareness of nociception occurs) may interpret the impulse as originating from a cutaneous or other recently injured region. Either of these regions may be distant to the structure that is currently damaged or inflamed. This phenomenon sometimes is called pain memory (Carpenter and Sutin, 1983; Wyke, 1987; Nolte, 1988).
The existence of pain referral between somatic structures has been documented for some time (Kellgren, 1938; Inman and Saunders, 1944; Hockaday and Whitty, 1967; McCall, Park, and O’Brien, 1979). The term somatic referred pain is used currently when discussing pain of somatic origin that is felt distant to the structure generating the nociception (Box 11-5). This type of pain is characterized as dull and aching, difficult to localize, and fairly constant in nature (Bogduk, 1997). For future reference, these characteristics of somatic referred pain are highlighted in Box 11-6.
Increased tenderness to deep palpation of the back muscles and hyperalgesia of innervated tissues may occur in areas of referred pain (Weinstein, 1988). An example of somatic referred pain is the pain arising from an inflamed Z joint, which may refer to the groin, buttock, greater trochanter of the femur, and posterior aspect of the thigh, extending to the knee and occasionally extending inferiorly to the leg’s posterior and lateral calf (Weinstein, 1988; Yukawa et al., 1997).
Takebayashi et al. (2001) recently identified an additional explanation for pain referring to regions innervated by nerves originating at higher spinal levels than the nerves that typically supply a particular pain generator. These investigators found that, in rats, some of the nerve fibers innervating spinal tissues originate from dorsal root ganglia several segments above those at the same segmental levels as the damaged tissues. In other words, some of the nerves innervating Mr. S.’s L4-5 IVD may originate from his L1 or L2 DRG and refer pain into his inguinal region (innervated by L1 and L2). In rats the fibers that eventually reach the higher dorsal root ganglia course from the pain generator of origin to gray rami communicantes at the level of the pain generator, ascend in the sympathetic chain, and then course through another gray communicating ramus that connects to an anterior primary division at the higher segmental level. From here the fibers pass through the APD, spinal nerve, DRG, and dorsal root at the higher level before synapsing in the more superior spinal cord segment. This work has been supported by the animal research (also rats) of others (Ohtori et al., 1999, 2001). Therefore the anulus fibrosus of the IVD is supplied by nerves originating from several segments and from both the left and right sides (Nakamura, 1996). Although more work in humans is needed to verify these animal studies, the results are consistent with the findings of clinical research showing that approximately 4.1% of patients with protrusion of the IVD at L4-5 or L5-S1 experience groin pain (Yukawa et al., 1997).
The threshold of nociceptors typically is lower than that of pain perception. This may result from the polysynaptic nature of the connections associated with most nociceptors. Many A-delta, and most C fibers synapse on one or more interneurons before reaching tract neurons of the ascending pathway that will transmit their information to the cortex, and each of these interneuronal connections is a site for possible modulation (inhibition). Another possible explanation of the difference in thresholds may result from central modulation of nociception via stimulation of mechanoreceptors (Greenspan, 1997). Consequently, activity of the muscles and the Z joints, as well as spinal manipulation of the Z joints, tends to decrease pain via a “gate control” type of mechanism (Melzack and Wall, 1965; Kirkaldy-Willis, 1988b). The work of Indahl et al. (1997) illustrates this point. In an elegant experiment using 29 adolescent pigs, these investigators stimulated the L3-4 IVD while recording muscle activity (by means of needle electromyography) from the multifidus and longissimus muscles. The stimulation of the IVD created an increased number of action potentials from the spinal muscles. The researchers then injected physiologic saline into the Z joint innervated by the same segmental level. The physiologic saline stretched the Z joint capsule. This resulted in significant decreased muscle activity. The authors concluded that stretching the Z joint capsule decreased multifidus muscle tightness (spasm) that was caused by pain arising from the IVDs (Indahl et al., 1997). Therefore if the pain was of somatic origin, Mr. S. might benefit most from treatment designed to promote activity and movement (Kirkaldy-Willis, 1988b). (See the section Modulation in the Spinal Cord for a more detailed explanation of the gate control theory.) Of related interest is that patients with LBP have been found to have decreased proprioception, as measured by standing and then four-point kneeling 10 times in 30 seconds (Gill and Callaghan, 1998). Consequently, increased activity, joint movement, and rehabilitative and proprioceptive training exercises also may help to improve Mr. S.’s joint position sense. However, recall that under certain circumstances mechanoreceptors can be sensitized to cause pain once tissue damage is well established, probably by means of central sensitization at the dorsal horn of the spinal cord (mechanical hyperalgesia) (Greenspan, 1997). Therefore precautions also should be taken to avoid further compromising any damaged tissue.
CENTRAL TRANSMISSION OF NOCICEPTION
Pain is the perception that results from the cerebral cortex’s interpretation of nociceptive input by a variety of CNS structures (Basbaum and Jessell, 2000). Some of the CNS structures that have been implicated in contributing to this process include the dorsal horn of the spinal cord; excitatory and inhibitory interneurons in the spinal cord; ascending pathways; reticular formation of the brain stem; thalamus; and several areas of the cerebral cortex, including the primary sensory cortex, cingulate gyrus, and insular cortex (Craig and Bushnell, 1994; Craig et al., 1996). The interconnections of these areas and subsequent integration of the information result in the components associated with the sensation of pain. These components include discriminatory qualities, emotions, attentiveness to the painful area, and reflex responses involving both the autonomic and endocrine systems (Haldeman, 1992).
The afferent fibers that convey nociception are group A-delta and C fibers. These fibers enter the dorsolateral tract of Lissauer, located at the tip of the cord’s dorsal horn. Some fibers continue directly into the gray matter of the dorsal horn, whereas their collateral branches ascend or descend numerous cord segments before entering the dorsal horn (see Fig. 11-3, A). The A-delta fibers convey nociception quickly and rapidly and terminate in lamina I and laminae V to VII. The group C fibers convey what is interpreted as a dull sensation of pain at a slow rate and terminate directly in lamina II and may indirectly (via interneurons) terminate in laminae V and VII. In addition, many of the neurons synapsing in lamina VII originate from both sides of the body, and may further contribute to the diffuse nature of many pain conditions (Basbaum and Jessell, 2000). The neurons that transmit the information to higher centers are located in various laminae of the gray matter (see Chapter 9). Surgical cordotomy procedures that relieve pain have shown that the most important fibers transmitting nociception to higher centers decussate in the ventral white commissure and then ascend in the anterolateral quadrant of the cord’s white matter (Hoffert, 1989). Alternative pathways also may be involved, although their course and function in humans remain unclear (Besson, 1988; Hoffert, 1989) (see Chapter 9).
Spinothalamic Tract
The response of the brain to painful stimuli is intricate and complex, and there are several pathways associated with Mr. S.’s LBP. Nociceptive information is conveyed to higher centers by tracts in the anterolateral quadrant of the spinal cord. Two major tracts conduct this information in the cord’s anterolateral quadrant, the spinothalamic tract (also called the neospinothalamic tract), and the spinoreticular tract. The spinothalamic tract conveys nociceptive information, which is perceived as sharp, pinpricklike pain, via fast conducting A-delta fibers. The integrity of this tract commonly is tested during a neurologic examination by asking the patient to differentiate the sensations of sharp versus dull. The cell bodies of the tract neurons are both nociceptive-specific and wide-dynamic-range neurons (sensitive to many types of stimuli) that are located in the dorsal horn primarily in laminae I and V to VII (Basbaum and Jessell, 2000). These axons decussate in the ventral white commissure within one or two segments of entry and ascend in the anterolateral white matter of the cord and through the brain stem. They synapse in the ventral posterior lateral nucleus and posterior nucleus, which comprise the lateral nuclear group of the thalamus (Fig. 11-4, A). From the thalamus, axons of the next order neurons course to the somesthetic region of the cortex, which is the postcentral gyrus and the posterior part of the paracentral lobule of the parietal lobe. As the axons of the spinothalamic tract ascend, body parts are represented in specific regions of the tract. This specific pattern is retained in the cerebral cortex, such that a specific area of cortex corresponds to the region of the body from which the sensory fibers originated. This cortical representation is called the sensory homunculus. The size of the body part represented on the homunculus reflects the amount of sensory innervation devoted to that body area. As mentioned, this unequal neuronal representation may be one reason that localization of sensations, such as pain, is more difficult in one region (e.g., back) than another (e.g., fingertips or lips). The information conveyed in the spinothalamic tract is processed in the region of the sensory homunculus. Here the nociception is perceived as pain that is acute, localized, and discriminatory.
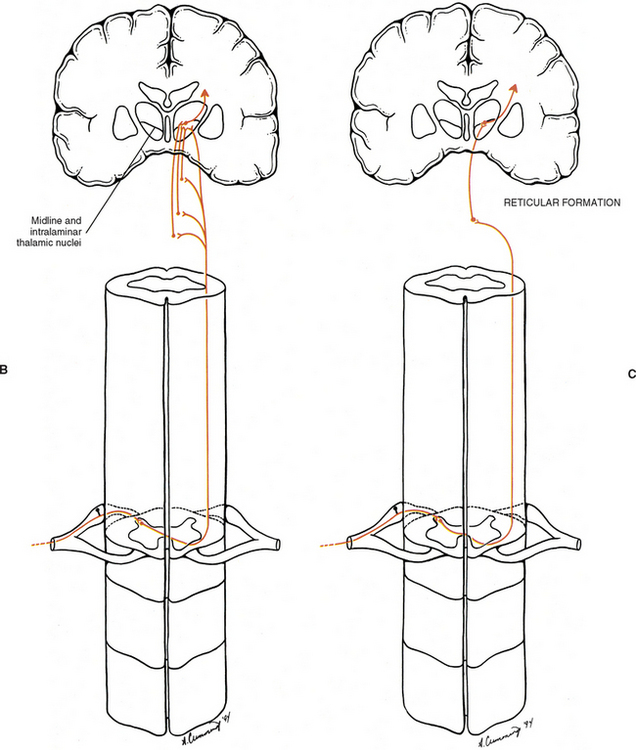
Spinoreticular Tract
The other tract that ascends in the anterolateral quadrant is the spinoreticular tract (Fig. 11-4, B and C). The cell bodies of the spinoreticular tract are located in laminae VII and VIII. The nociceptive input to these neurons is polysynaptic; therefore these neurons are likely involved with more complex response properties of pain. The axons of these neurons ascend to the reticular formation of the brain stem and to the thalamus. The reticular formation is a complex network of neurons located throughout the core of the brain stem. It has numerous functions and is a major component of the ascending reticular activating system (ARAS), along with the thalamus and cerebral cortex. The ARAS provides the circuitry through which arousal and attentiveness are maintained. The tract neurons synapsing in the reticular formation form complex connections within this region and subsequently project to brain stem nuclei, the hypothalamus, and the midline and intralaminar nuclei of the thalamus. These latter nuclei are part of the medial nuclear group of the thalamus. Subsequent thalamic projections course to widespread, nonspecific areas of the cerebral cortex. In addition, the paleospinothalamic tract (also known as the spinoreticulothalamic tract) contributes collateral branches to the reticular formation as it ascends through the brain stem to the midline and intralaminar thalamic nuclei. The spinoreticular tract and spinoreticulothalamic fibers are not somatotopically organized. The nociceptive information conveyed in these fibers also may be involved with the generation of chronic pain and the qualities associated with that sensation. The focusing of the individual’s attention on the painful area is most likely a function of the ARAS.
Dimensions of Pain
Pain appears to be of two dimensions. One is a sensory portion that is involved with identifying the location, intensity, quality, and other characteristics of the pain for an appropriate motor response. The other is the dimension of affect and motivation. This is the subjective awareness of the unpleasantness of the pain: the desire (motivation) to terminate, reduce, or escape the stimulus. It includes the emotions involved with present, short-term, or long-term implications of the pain (i.e., interference with one’s life) and the cognitive processes of how to cope with the pain. Pain is a complex sensation, and multiple pathways that ascend both in series and in parallel terminate in numerous brain stem and cortical regions to participate in its processing. The pathway that serves the dimension of pain sensation is the (neo)spinothalamic pathway, sometimes called the lateral and more direct pathway. As discussed, this pathway projects to the somatosensory cortex, which in turn projects to other cortical areas such as the posterior parietal lobe and insular cortex. From these two areas, projections continue to other areas (e.g., the amygdala and anterior cingulate cortex) that have reciprocal connections with the prefrontal cortex. This pathway may be involved not only with the rudimentary aspects of pain such as intensity, quality, and location; but also with integrating numerous somatosensory inputs. The integration of these inputs provides an overall feeling of the seriousness or threat of the pain stimulus to the body and self (Price, 2000). The aspect of affect and motivation to pain sensation is served by numerous pathways sometimes collectively called the medial pathway (Cross, 1994; Hudson, 2000; Sewards and Sewards, 2002). The spinoreticular and spinoreticulothalamic tracts (see previous section) and spinomesencephalic and spinohypothalamic tracts (see Chapter 9) terminate in brain stem nuclei, the thalamus, and hypothalamus (Price, 2000). Processing at these levels may produce elementary aspects of pain behavior by involving autonomic activation, escape responses, arousal, and fear. These attributes occur early in pain-processing stages when fear, defensive behavior, and visceral responses occur. The thalamic nuclei of this pathway (midline and intralaminar) project to the limbic system, which allows an individual to perceive a sensation as being uncomfortable, aching, or hurting (Haldeman, 1992). The component of the limbic system receiving this input is the anterior cingulate cortex (see Fig. 9-14). This region is considered to be a major cortical area for processing the emotional (motivational) component of pain (Hudson, 2000; Price, 2000; Raineville, 2002; Sewards and Sewards, 2002). The anterior cingulate cortex is indirectly and directly connected with many other areas including the posterior cingulate cortex, amygdala (which functions in the memory aspects of painful experiences), insular cortex (which functions in the autonomic component of the entire painful episode), parietal cortex, and prefrontal cortex. Because of these interactions, the anterior cingulate cortex may be a region that integrates pain information received from sensory cortices concerning recognition and threat of the painful experience with information received from the prefrontal cortex. This integration is considered important for individuals like Mr. S. to plan how to respond and cope with the painful experience.
IMPORTANT TERMS AND GENERAL CONCEPTS RELATED TO PAIN
The sensations that are generally thought of as painful are all perceptions by the cerebral cortex, and therefore subjective. These perceptions are based on previous experience and understanding that something injurious has occurred to the body. Most painful conditions experienced or described by patients have a physiologic basis, and understanding the mechanisms associated with the stimulation of nociceptors and the subsequent perception of pain is important to properly diagnose and treat any injury. The following section contains a list of many of the common terms used in further describing and differentiating diagnoses related to pain. Many of the terms used to describe, define, or classify pain for diagnostic purposes were developed specifically for use in clinical practice, hence the need here for additional physiologic descriptions to provide information related to the mechanisms associated with a particular term. Each term is followed immediately (in quotes) by the current definition as described by the International Association for the Study of Pain (Merskey and Bogduk, 1994), a physiologic or anatomic description for that term, and finally, a brief description, where appropriate, relating to Mr. S.
Terms Related to Pain
Nociceptor.
“A receptor preferentially sensitive to a noxious stimulus or to a stimulus which would become noxious if prolonged.” All nociceptors are specialized primary sensory neurons whose cell bodies are located in the dorsal root or trigeminal ganglia. The primary sensory endings are usually in the periphery, and they transmit their information into the CNS via central processes of either the dorsal root ganglia or trigeminal ganglia. There are three major classes of nociceptors: thermal, mechanical, and polymodal. Nociceptors transduce (convert) the mechanical, thermal, and chemical signals into action potentials that synapse in the dorsal horn (or spinal nucleus of the trigeminal nerve) onto second-order “pain” pathway neurons. The pain pathways eventually reach the cortex and are interpreted by the CNS as pain (see Central Transmission of Nociception for a detailed discussion of these pathways). Thermal nociceptors are activated by extreme temperatures (>45° C or <5° C) that could potentially damage the body. Mechanical nociceptors are activated by intense pressure or stretch. Polymodal nociceptors are activated by high-intensity mechanical, chemical, or thermal stimuli (Basbaum and Jessell, 2000). In addition, the viscera and many other tissues (including spinal tissues) contain “silent nociceptors” that normally are not activated by noxious stimuli. These receptors can be dramatically altered by inflammation or chemical insults, resulting in a significant lowering of their firing thresholds and a concomitant increase in their firing rate. Therefore these “silent nociceptors” may contribute to the development of secondary hyperalgesia or central sensitization (described later in this section).