Pain Management
KEY CONCEPTS
![]() It is important, whenever possible, to ask patients if they have pain, to identify the source of pain, and to assess the characteristics of the pain.
It is important, whenever possible, to ask patients if they have pain, to identify the source of pain, and to assess the characteristics of the pain.
![]() Doses must be individualized for each patient and administered for an adequate duration of time. Around-the-clock regimens should be considered for acute and chronic pain. As-needed regimens should be used for breakthrough pain or when acute pain displays wide variability and/or has subsided greatly.
Doses must be individualized for each patient and administered for an adequate duration of time. Around-the-clock regimens should be considered for acute and chronic pain. As-needed regimens should be used for breakthrough pain or when acute pain displays wide variability and/or has subsided greatly.
![]() For chronic pain that has a neuropathic component, anticonvulsants, topical analgesics, tricyclic antidepressants, serotonin–norepinephrine reuptake inhibitors, and opioids should be considered based on evidence-based recommendations when available.
For chronic pain that has a neuropathic component, anticonvulsants, topical analgesics, tricyclic antidepressants, serotonin–norepinephrine reuptake inhibitors, and opioids should be considered based on evidence-based recommendations when available.
![]() Oral analgesics are preferred over other dosage forms whenever feasible, but it is important to adjust the route of administration to the needs of the patient.
Oral analgesics are preferred over other dosage forms whenever feasible, but it is important to adjust the route of administration to the needs of the patient.
![]() Equianalgesic doses are useful as a guide when converting from one agent to another, but further dose titration usually is required to achieve treatment goals.
Equianalgesic doses are useful as a guide when converting from one agent to another, but further dose titration usually is required to achieve treatment goals.
![]() Patients taking analgesics should be monitored for response and side effects, particularly sedation and constipation associated with the opioids.
Patients taking analgesics should be monitored for response and side effects, particularly sedation and constipation associated with the opioids.
![]() Care should be taken to identify and avoid potential drug–drug interactions with analgesics when possible, as increased adverse effects may occur (e.g., opioids and benzodiazepines).
Care should be taken to identify and avoid potential drug–drug interactions with analgesics when possible, as increased adverse effects may occur (e.g., opioids and benzodiazepines).
![]() Whenever possible, a multidisciplinary approach and nonpharmacologic strategies should be used.
Whenever possible, a multidisciplinary approach and nonpharmacologic strategies should be used.
![]() Etiology of pain may not always be identifiable.
Etiology of pain may not always be identifiable.
If we know that pain and suffering can be alleviated, and do nothing about it, then we ourselves, become the tormentors.
Primo Levi1
Humans have always known and sought relief from pain.2 Today, pain’s impact on society still is great, and indeed pain complaints remain a primary reason patients seek medical advice.3
Regrettably, many healthcare providers do not receive adequate training in this area, and new information is not widely disseminated and/or understood. Clearly, pain management is enhanced when a multidisciplinary approach is applied. Thus, understanding the pathophysiology of pain therapy and maintaining a working knowledge of pain regimens are important factors in addressing pain control.
DEFINITION
The accepted current definition of pain is: “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”4 Pain often is so subjective, however, that many clinicians define pain as whatever the patient says it is. The best care is achieved when the patient comes first.
EPIDEMIOLOGY
Data presented in the recently released Institute of Medicine report, “Relieving Pain in America” suggests that greater than 100 million persons in the United States live with chronic pain.5 Given that greater than 50% of persons with low back pain in the previous 3 months reported interference with basic and complex activity, it is not surprising that the estimated economic burden of just chronic pain alone exceeds 500 billion dollars (US) annually.5 In 1 year, an estimated 25 million Americans will experience acute pain due to injury or surgery, and one third of Americans will experience severe chronic pain at some point in their lives.3 Unfortunately, despite much public attention, considerable focused education, and a number of consensus guidelines, pain often remains inadequately or inappropriately treated.3,5–10
PHYSIOLOGY AND PATHOPHYSIOLOGY
The pathophysiology of pain involves a complex array of neural networks in the brain that are acted on by afferent stimuli to produce the experience we know as pain. It can be physiologic and protective (nociceptive) or pathophysiologic and harmful (e.g., neuropathic).11
Nociceptive Pain
Nociceptive pain, which can be considered protective and physiologic,11 typically is classified as either somatic (arising from skin, bone, joint, muscle, or connective tissue) or visceral (arising from internal organs such as the large intestine or pancreas).11 Whereas somatic pain often presents as throbbing and well localized, visceral pain can manifest as pain feeling as if it is coming from other structures (referred) or as a more localized phenomenon.11 We can think of nociception in terms of transduction, transmission, perception, and modulation.11
Transduction
The first step leading to the sensation of pain is stimulation of free nerve endings known as nociceptors. These receptors are found in both somatic and visceral structures. They distinguish between noxious and innocuous stimuli, and they are activated and sensitized by mechanical, thermal, and chemical impulses.11 The underlying mechanism of these noxious stimuli (which in and of themselves may sensitize/stimulate the receptor) may be the release/activation of bradykinins, nerve growth factor, prostaglandins, histamine, interleukins, tumor necrosis factor α, serotonin, and substance P (among others) that sensitize and/or activate the nociceptors.11,12 Receptor activation (which also involves voltage-gated sodium channels) leads to action potentials that are transmitted along afferent nerve fibers to the spinal cord11,12 (Fig. 44-1).
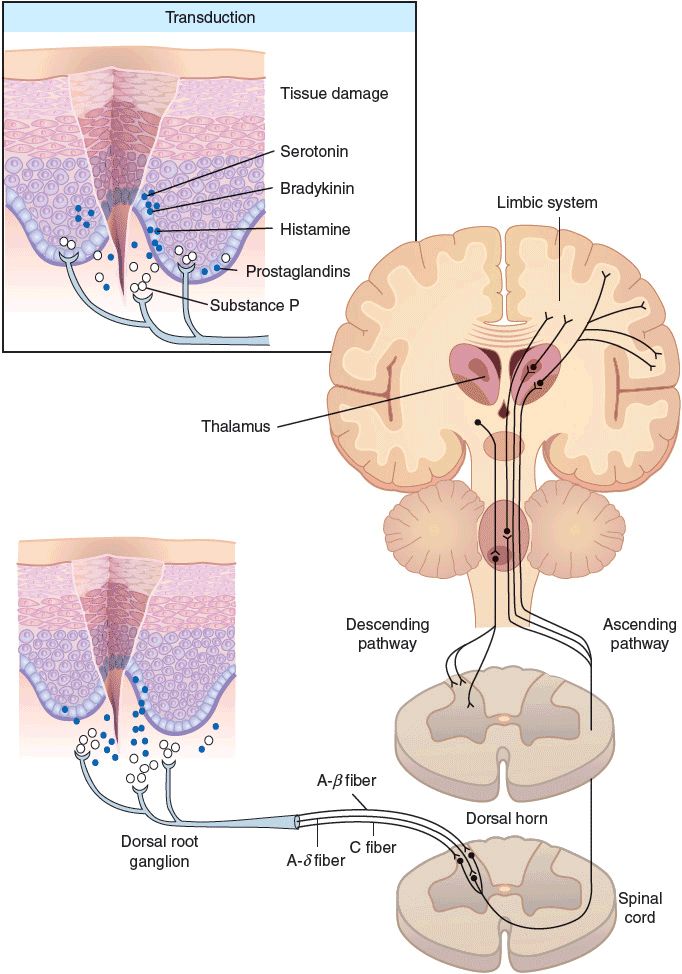
FIGURE 44-1 Schematic representation of nociceptive pain. (Used with permission from, Pasero C, Portenoy R. Neurophysiology of pain and analgesia and the pathophysiology of neuropathic pain. In: McCaffery M, Pasero C, eds. Pain Assessment and Pharmacologic Management. St. Louis: Mosby, 2011:4–5, Figure 1-2.)
Transmission
Nociceptive transmission takes place in A-δ and C-afferent nerve fibers.11 Stimulation of large-diameter, sparsely myelinated A-δ fibers evokes sharp, well-localized pain, whereas stimulation of unmyelinated, small-diameter C fibers produces aching, poorly localized pain.11
These afferent, nociceptive pain fibers synapse in various layers (laminae) of the spinal cord’s dorsal horn, releasing a variety of neurotransmitters, including glutamate, substance P, and aspartate.13 The complex array of events that influence pain can be explained in part by the interactions between neuroreceptors and neurotransmitters that take place in this synapse. For example, by stimulating sensory myelinated fibers that intraconnect in the dorsal horn with pain fibers, nonnoxious stimuli can have an inhibitory effect on pain transmission. These pain-initiated processes reach the brain through a complex array of ascending spinal cord pathways, which include the spinothalamic tract.13 Information other than pain is also carried along these pathways. Thus, pain is influenced by many factors supplemental to nociception and precludes simple schematic representation. It is postulated that the thalamus acts as a relay station, as these pathways ascend and pass the impulses to central structures where pain can be processed further.14
Perception
At this point in transmission, pain is thought to become a conscious experience that takes place in higher cortical structures. The physiology surrounding perception is complex and not well understood, but we know cognitive and behavioral functions can modify pain. Thus relaxation, distraction, meditation, and guided mental imagery may strongly influence pain perception11 and decrease pain. In contrast, a change in our neurobiochemical makeup that results in states such as depression or anxiety may worsen pain.
Modulation
The body modulates pain through a number of complex processes. One, known as the endogenous opiate system, consists of neurotransmitters (e.g., enkephalins, dynorphins, and β-endorphins) and receptors (e.g., μ, δ, and κ) that are found throughout the central and peripheral nervous system (CNS and PNS).11,15 Like exogenous opioids, endogenous peptides bind to opioid receptor sites and modulate the transmission of pain impulses.11 Other receptor types also can influence this system. Blockade of N-methyl-D-aspartate (NMDA) receptors, found in the dorsal horn, may increase the μ-receptors’ responsiveness to opiates.15
The CNS also contains a highly organized descending system for the control of pain transmission. This system, which at least partially originates in the periaqueductal gray region of the midbrain, can inhibit synaptic pain transmission at the dorsal horn via modulation of the raphe nuclei in the brainstem.11,16 Important neurotransmitters here include endogenous opioids, serotonin, norepinephrine, and γ-aminobutyric acid (GABA).11,16
Pathophysiologic Pain
Pathophysiologic pain is distinctly different from nociceptive pain in that it becomes disengaged from noxious stimuli or healing11 and often is described in terms of chronic pain. This type of pain is a result of damage or abnormal functioning of the PNS and/or CNS.11 A number of pain syndromes (e.g., postherpetic neuralgia, diabetic neuropathy, fibromyalgia, irritable bowel syndrome, sympathetic induced pain, chronic headaches, and some noncardiac chest pain) fall into this category. These pain syndromes are often under-recognized and difficult to treat. In addition, the pain reported often is not commensurate with physical exam findings or imaging results.
The mechanism responsible for pain of this nature may be the nervous system’s endogenous dynamic nature. Nerve damage or certain disease states evoke both peripheral (e.g., alteration in nociceptive nerve fibers sensitivity, alterations in sodium channels, collateral sprouting of nerve fibers) and central (e.g., hyperexcitability of central neurons or central sensitization, NMDA receptor activation, central disinhibition) mechanisms leading to these changes.11,17 Pain circuits rewire themselves both anatomically and biochemically.18 This produces a mismatch between pain stimulation and inhibition and a potential progressive increase in the discharge of dorsal horn neurons.18
Clinically, patients present with episodic or continuous pain transmission (often described as burning, tingling, shock like, or shooting) exaggerated painful response to normally noxious stimuli (hyperalgesia), and/or painful response to normally nonnoxious stimuli (allodynia).3,18 This change over time may help to explain why this type of pain often manifests long after the actual nerve-related injury or when no actual injury is identified.
CLASSIFICATION OF PAIN
It is helpful in understanding pain to subdivide the presenting symptoms into acute pain, chronic pain, and cancer pain.
Acute Pain
Acute pain can be a useful physiologic process, warning individuals of disease states and potentially harmful situations. Unfortunately, severe, unremitting, undertreated acute pain, when it outlives its biologic usefulness, can produce many deleterious effects. Aside from unnecessary suffering, untreated and undertreated acute pain has also been shown to increase one’s risk for the development of chronic pain syndromes.19 Acute pain is usually nociceptive in nature with common causes, including surgery, acute illness, trauma, labor, and medical procedures.3
Chronic Pain
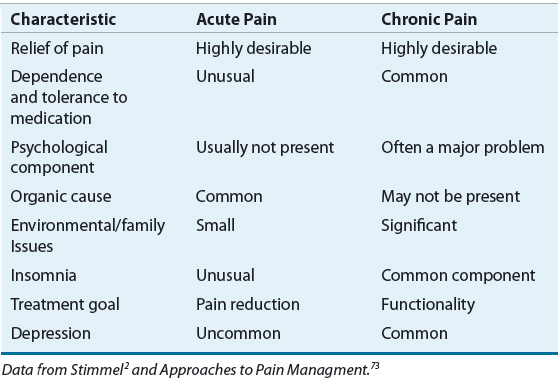
Under normal conditions, acute pain subsides quickly as the healing process decreases the pain-producing stimuli; however, in some instances, pain persists for months to years, leading to a chronic pathophysiologic pain state with features quite different from those of acute pain (Table 44-1).3 Chronic pain can be classified as either being associated with cancer (cancer pain) or from noncancer etiologies (chronic noncancer pain). It often is a result of changes to nerve function and transmission thus making treatment difficult.20
TABLE 44-1 Characteristics of Acute and Chronic Pain
Cancer Pain
Pain associated with potentially life-threatening conditions is often called malignant pain or in the case of cancer, cancer pain.3 This type of pain includes both chronic and acute (e.g., breakthrough pain) components and often has multiple etiologies. It is pain caused by the disease itself (e.g., tumor invasion, organ obstruction), treatment (e.g., chemotherapy, radiation, and surgical incisions), or diagnostic procedures (e.g., biopsy).3
CLINICAL PRESENTATION
A patient-oriented approach is essential, and evaluation methods should not differ from those used in other medical conditions.21
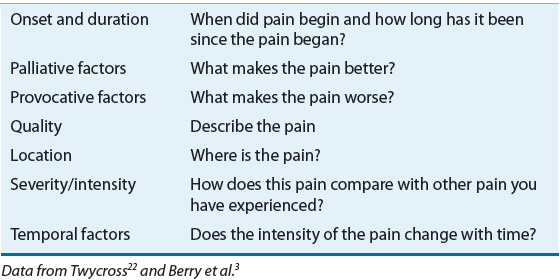
![]() Therefore, a comprehensive history and physical examination are imperative to evaluate underlying diseases and possible other contributing factors.2 This includes asking if the patient has pain and identifying the source of pain when possible, however, the absence of a discreet etiology should not preclude pain treatment.2 A baseline characterization of pain can be obtained by assessing the attributes outlined in Table 44-2.22
Therefore, a comprehensive history and physical examination are imperative to evaluate underlying diseases and possible other contributing factors.2 This includes asking if the patient has pain and identifying the source of pain when possible, however, the absence of a discreet etiology should not preclude pain treatment.2 A baseline characterization of pain can be obtained by assessing the attributes outlined in Table 44-2.22
TABLE 44-2 Pain Attributes
CLINICAL PRESENTATION Pain
TREATMENT
Desired pain management outcomes include both nonpharmacologic and pharmacologic strategies.
Desired Outcomes
The primary goal of pain treatment depends on the type of pain present and should be tailored to individual patients and circumstances.![]()
![]() For example, in acute pain, rapid pain relief or reduction in pain intensity is usually the desired target. In comparison, the goal in chronic noncancer pain is to improve or maintain the patient’s level of day-to-day functioning, decrease the rate of physical deterioration, decrease pain perception, improve the patient’s sense of well-being, improve family and social relationships, and decrease dependency on drug therapy.2 And finally in cancer pain or other forms of malignant pain, the goal is to provide patients with adequate pain relief that allows them to tolerate diagnostic and therapeutic manipulation and permit the patient to function at a level that will allow freedom of movement and choice.23
For example, in acute pain, rapid pain relief or reduction in pain intensity is usually the desired target. In comparison, the goal in chronic noncancer pain is to improve or maintain the patient’s level of day-to-day functioning, decrease the rate of physical deterioration, decrease pain perception, improve the patient’s sense of well-being, improve family and social relationships, and decrease dependency on drug therapy.2 And finally in cancer pain or other forms of malignant pain, the goal is to provide patients with adequate pain relief that allows them to tolerate diagnostic and therapeutic manipulation and permit the patient to function at a level that will allow freedom of movement and choice.23
Nonpharmacologic Therapy
Various nonpharmacologic therapies have been found to be beneficial in the management of acute and chronic pain, including physical manipulation, application of heat or cold, massage, biofeedback, cognitive behavioral therapy, relaxation, acupuncture, and exercise.24–26 Spinal cord stimulators have also been found to be somewhat beneficial in some chronic pain conditions.27
Some commonly used nonpharmacologic therapies, including TENS (transcutaneous electrical nerve stimulation) and lumbar supports in back pain, have limited evidence or have been shown to be ineffective in the management of chronic pain.26
Simple interventions (e.g., education or introductory information about expected discomfort or pain after certain procedures) reduce patient distress and greatly reduce postprocedure suffering.28 Some psychological techniques, including cognitive-behavioral therapy, relaxation training, imagery, and hypnosis, have proven effective in numerous types of pain, including postprocedure pain, low back pain, and cancer-related pain.23,25,26,28 Nonpharmacologic therapies should be considered when possible.
Pharmacologic Treatment
Pharmacologic treatment is often considered the cornerstone of pain management.
Nonopioid Agents
Analgesia should be initiated with the most effective analgesic agent having the fewest side effects. Acetaminophen and nonsteroidal antiinflammatory drugs (NSAIDs) often are preferred first-line therapies, in the treatment of mild-to-moderate pain (Table 44-3). The exact mechanism of acetaminophen is not completely understood. NSAIDs inhibit formation of varying prostaglandins produced in response to noxious stimuli, thereby decreasing the pain impulses received by the CNS.29 Acetaminophen is indicated as a first-line therapy in some pain-related disease states, such as osteoarthritis. NSAIDs may be particularly useful in the management of cancer-related bone pain and for short-term relief in the management of chronic low back pain.23,30
TABLE 44-3 Adult FDA-Approved Nonopioid Analgesics
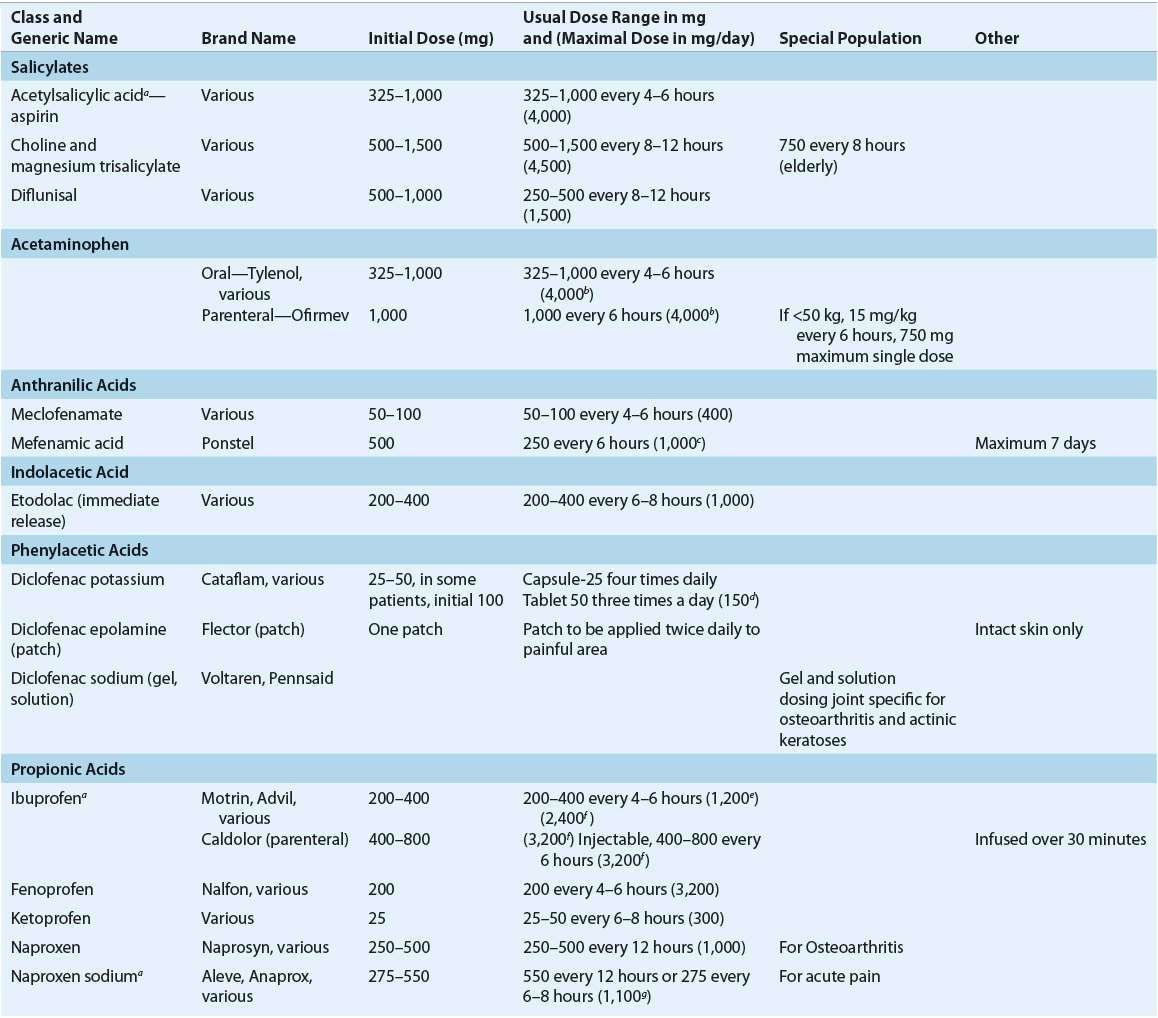
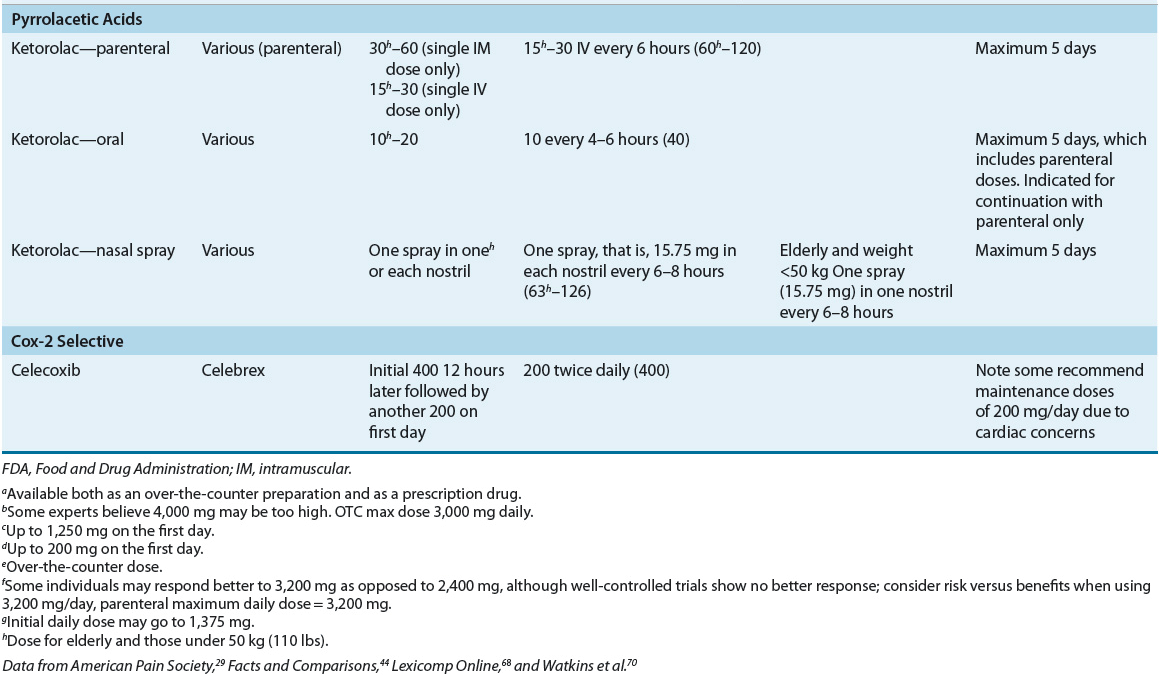
Studies comparing the efficacy of individual NSAIDs have failed to identify greater efficacy of any NSAID compared to any other. Therefore, the choice of a particular agent often depends on availability, cost, pharmacokinetics, pharmacologic characteristics, and the side-effect profile. Because of the large interpatient variability in response to individual NSAIDs, it is considered rational therapy to switch to another member of this class if there is inadequate response after a sufficient therapeutic trial of any single agent.29 The duration of a sufficient trial has not been well defined; however, typically, a NSAID should be continued for a minimum of 1 month prior to evaluating the need to switch agents. Chronic use of NSAIDs, including selective inhibitors of cyclooxygenase 2 (COX-2 inhibitors), may be limited by adverse effects, including GI, renal, and cardiac effects. Topical NSAIDs may offer similar efficacy as oral NSAIDs in some patients with improved safety and tolerability.31 Patient selection is critical to ensure optimal benefit from NSAID therapy while minimizing potential adverse effects.
Opioid Agents
Opioids are often the next logical step in the management of acute pain and cancer-related chronic pain. They also may be an effective treatment option in the management of chronic noncancer pain; however, this continues to be somewhat controversial. Many times a trial of opioids is warranted, but such a trial should not be done without a complete assessment of the pain complaint, including an assessment of the patient’s functionality and risk factors for opioid misuse and abuse.32
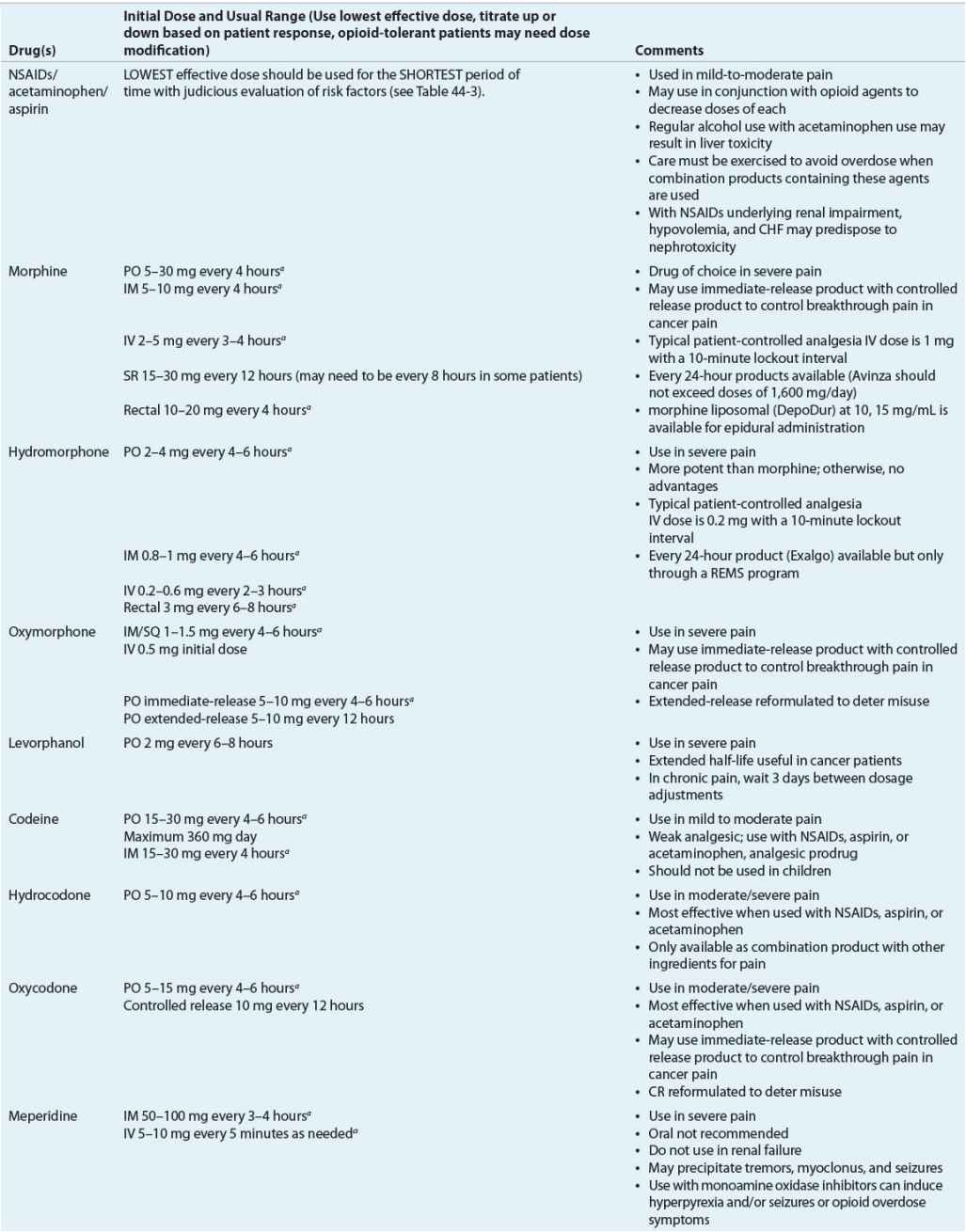
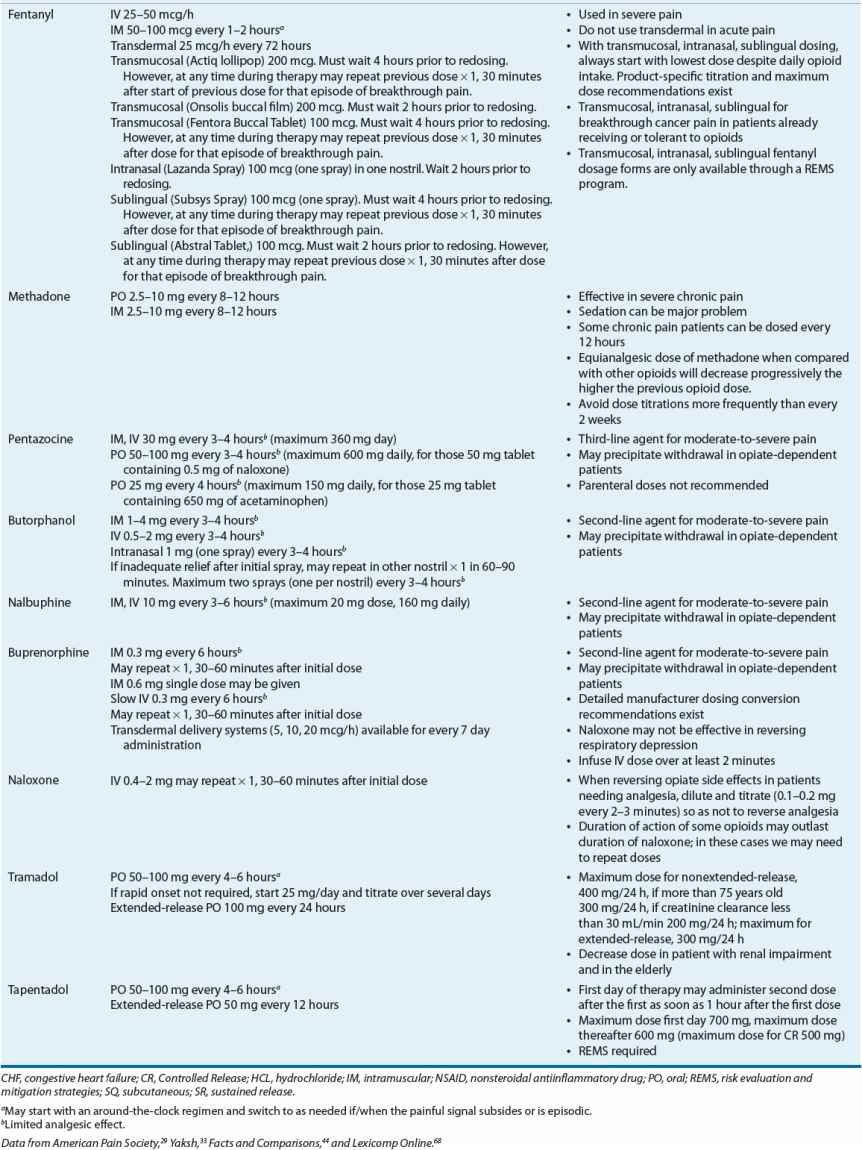
The classification of these agents, their equianalgesic doses, relative histamine-releasing characteristics, pharmacokinetics, and dosing guidelines are outlined in Tables 44-4 and 44-5. Opioid choice should be based on patient acceptance; analgesic effectiveness; and pharmacokinetic, pharmacodynamic, and side-effect profiles (Tables 44-4 and 44-6).
TABLE 44-4 Opioid Analgesics, Central Analgesics, Opioid Antagonist
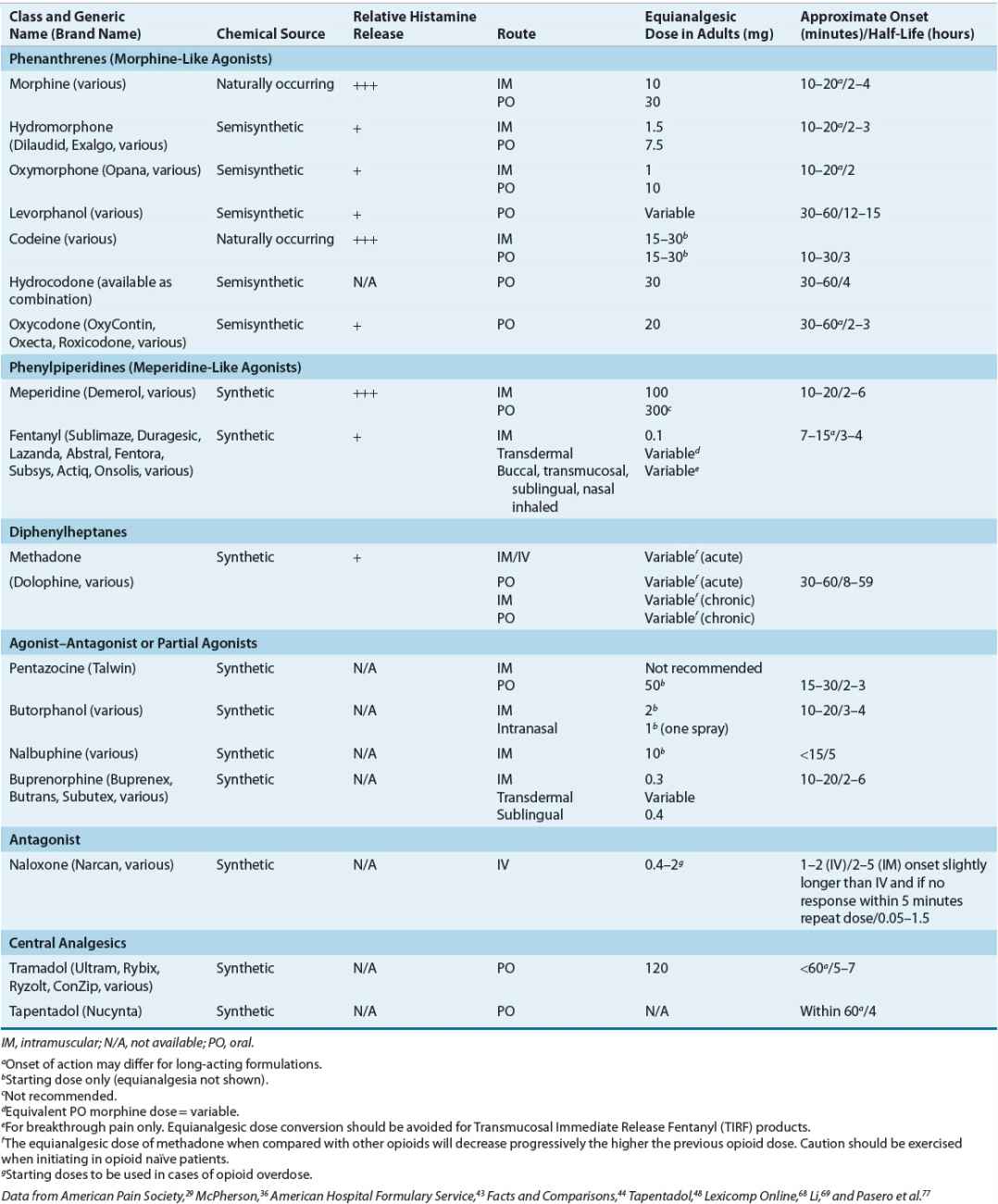
TABLE 44-6 Analgesic Drug Monitoring
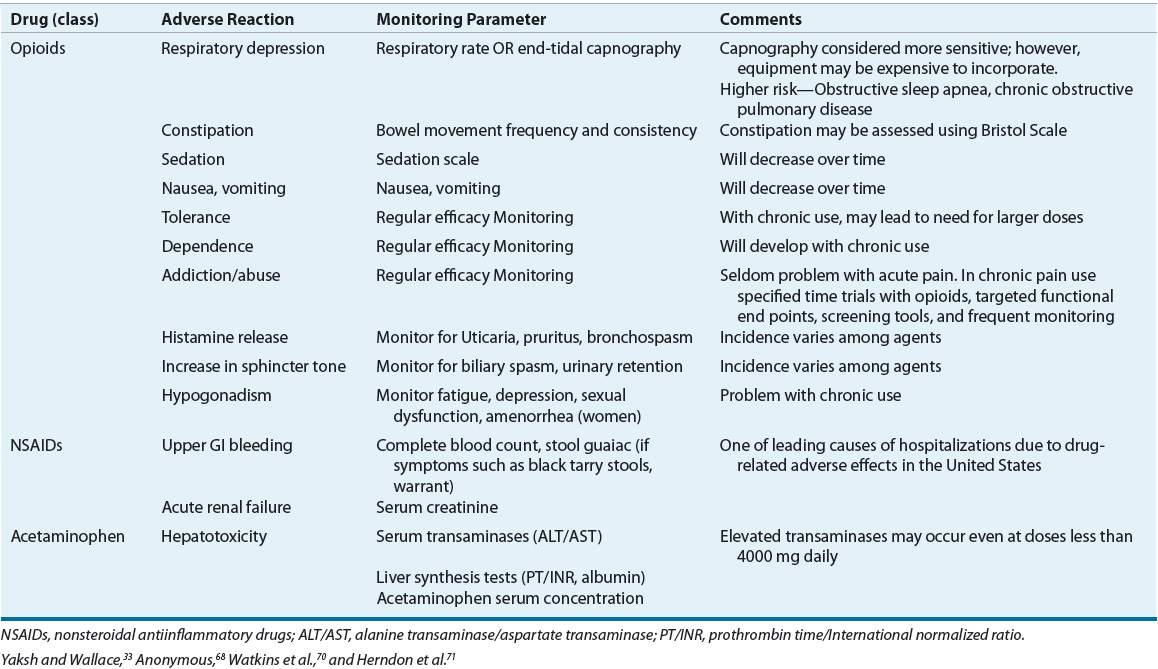
The pharmacologic activity of opioids depends on their affinity for and action at central and peripheral opiate receptors.33 Therapeutic activities and side effects range from those exhibited by the opiate agonists (e.g., morphine) to those seen with the opiate antagonists (e.g., naloxone). Partial agonists and antagonists (e.g., nalbuphine) compete with agonists for opiate receptor sites and, depending on the inherent agonist and antagonist properties, exhibit mixed agonist–antagonist activity.33 This may result in analgesia with fewer undesirable side effects. Efficacy and side effects also may further differ among agents because of receptor subtype variability.34 This μ-receptor subtype variability may explain why some patients respond differently to certain opioids, specifically μ-receptor agonists.34
The effects of the opioid analgesics are relatively selective, and at normal therapeutic concentrations, do not affect other sensory modalities.33 While sensations of touch and proprioception are preserved; undesirable side effects may increase as the dose is escalated (Table 44-6).33 Patients in severe pain may receive high doses of opioids with no unwanted side effects, but as the pain subsides, even very low doses may not be tolerated.35 Frequently, when opioids are administered, pain is not eliminated, but its unpleasantness is decreased.33 Patients report that although their pain is still present, it no longer bothers them.
Opioids share related pharmacologic attributes and exert a profound effect on the CNS and GI tract.33 Mood changes, sedation, nausea, vomiting, decreased GI motility, constipation, respiratory depression, dependence, and tolerance are evident in varying degrees with all agents.3,33 Tolerance to side effects (except to constipation) often develops over time.3 Some differences exist between the opioids in regards to incidence of side effects, which may assist in selection of the most appropriate agent. ![]() The route of administration depends on individual patient needs, with the oral route being preferred. However, the onset of analgesic effects for oral medications is approximately 45 minutes, and the peak effect usually occurs 1 to 2 hours after administration.29 This delay must be a consideration when immediate relief is needed in the management of acute pain. Therefore, in some scenarios, such as acute severe pain (e.g., pain crisis) or when the patient is unable to take oral medications, alternative routes of therapy (e.g., IV) may be preferred. The relative potency, defined by the equianalgesic dose, of opioids differs greatly (Table 44-4).
The route of administration depends on individual patient needs, with the oral route being preferred. However, the onset of analgesic effects for oral medications is approximately 45 minutes, and the peak effect usually occurs 1 to 2 hours after administration.29 This delay must be a consideration when immediate relief is needed in the management of acute pain. Therefore, in some scenarios, such as acute severe pain (e.g., pain crisis) or when the patient is unable to take oral medications, alternative routes of therapy (e.g., IV) may be preferred. The relative potency, defined by the equianalgesic dose, of opioids differs greatly (Table 44-4).![]() Equianalgesic dose tables are often based on single-dose studies without regard for patient variability and should be used only as a guide.36
Equianalgesic dose tables are often based on single-dose studies without regard for patient variability and should be used only as a guide.36
True opioid allergies are rare, but Table 44-4 also can be used when treating a patient who is allergic to opiates. Most reactions to opioids, such as itching or rash, are due to the associated histamine release from cutaneous mast cells, not a true allergic or immunoglobulin-E (IgE) or T-cell response.37 Although caution is always advised, a decrease in potential cross-sensitivity is thought to exist when moving from one opioid structural class to another.37 The classes are phenanthrenes (morphine-like agonists), phenylpiperidines (meperidine-like agonists), and diphenylheptanes (methadone-like agonists). When considering cross-sensitivity, the mixed agonist–antagonist and partial agonists class acts much like the morphine-like agonists.37
![]()
![]() In the initial stages of acute pain, analgesics should be given around the clock. This should commence after administering a typical starting dose and titrating up or down, depending on the patient’s degree of pain and demonstrated side effects (e.g., sedation).29 As-needed schedules often produce wide swings in analgesic plasma concentrations that create wide swings in pain and sedation. This may initiate a vicious cycle where increasing amounts of pain medications are needed for relief.
In the initial stages of acute pain, analgesics should be given around the clock. This should commence after administering a typical starting dose and titrating up or down, depending on the patient’s degree of pain and demonstrated side effects (e.g., sedation).29 As-needed schedules often produce wide swings in analgesic plasma concentrations that create wide swings in pain and sedation. This may initiate a vicious cycle where increasing amounts of pain medications are needed for relief.![]() As the painful state subsides and the need for medication decreases, as-needed schedules may be appropriate. As-needed schedules also may be useful in patients who present with pain that is intermittent or sporadic in nature (Fig. 44-2). When opioids are used in the management of persistent chronic pain, around-the-clock administration schedules should be utilized. As-needed or prn opioids should be used in conjunction with around-the-clock regimens and when patients experience breakthrough pain. Breakthrough pain is a brief, transitory, exacerbation of moderate to severe pain typically occurring in patients with underlying persistent pain that may otherwise be controlled.36
As the painful state subsides and the need for medication decreases, as-needed schedules may be appropriate. As-needed schedules also may be useful in patients who present with pain that is intermittent or sporadic in nature (Fig. 44-2). When opioids are used in the management of persistent chronic pain, around-the-clock administration schedules should be utilized. As-needed or prn opioids should be used in conjunction with around-the-clock regimens and when patients experience breakthrough pain. Breakthrough pain is a brief, transitory, exacerbation of moderate to severe pain typically occurring in patients with underlying persistent pain that may otherwise be controlled.36
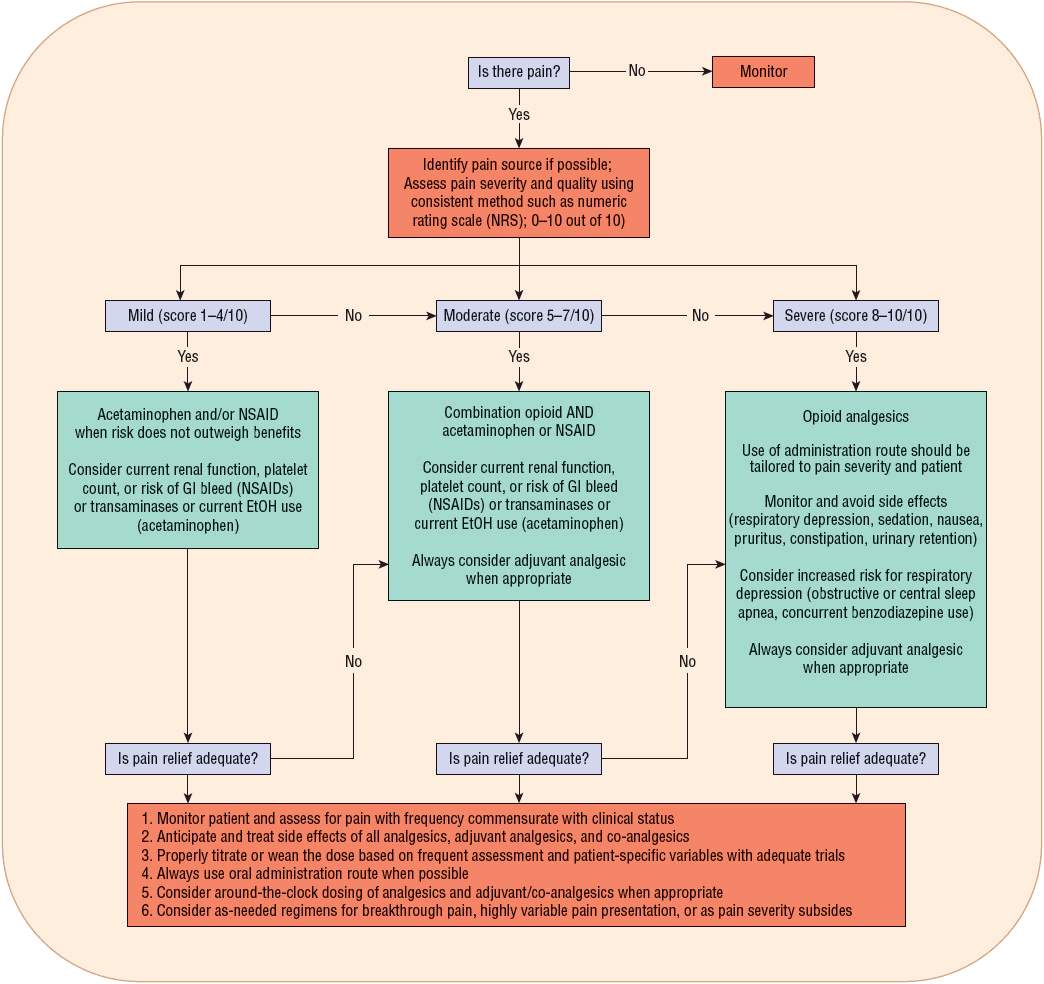
FIGURE 44-2 Algorithm for acute pain. (Data modified from Omnicare, Inc., Acute Pain Pathway.)