Pain
Learning Objectives
After studying this chapter, the student is expected to:
3. Relate the methods of pain control to the gate-control theory.
4. Discuss the signs and symptoms of pain in adults and young children.
5. Compare referred and phantom pain.
6. Explain the factors that may alter pain perception.
7. Compare acute and chronic pain.
8. Discuss the types of headache.
Key Terms
afferent fibers
analgesic
bradykinin
cordotomy
dermatome
efferent
endogenous
histamine
intractable
ischemia
neurotransmitter
nociceptors
opioids
parenterally
prostaglandin
reticular activating system (RAS)
reticular formation
rhizotomy
sedatives
substance P
tachycardia
Pain is an unpleasant sensation, a feeling of discomfort resulting from stimulation of pain receptors in the body when tissue damage occurs or is about to occur. Pain is a body defense mechanism and is a warning of a problem, particularly when it is acute. It is difficult to define because it can have many variable characteristics, and it is a subjective feeling, impossible to accurately measure. However, subjective scales have been developed to facilitate comparison of pain levels over time. In cases of trauma, the danger may be obvious, but in other situations the cause may be hidden deep inside the body. Pain involves very complex mechanisms, many of which are not totally understood by scientists or health care workers.
Etiology and Sources of Pain
Pain stimuli may occur for many reasons. Pain may be felt because of inflammation, infection, ischemia and tissue necrosis, stretching of tissue, chemicals, or burns. In skeletal muscle, pain may result from ischemia or hemorrhage. Many organs such as the liver, kidney, or brain are characterized by pain receptors in the covering capsule, and pain is felt when the capsule is stretched by inflammation. Stretching of tendons, ligaments, and joint capsules also elicits pain; these effects may occur secondary to inflammation or muscle spasm to guard a joint or painful body part. In the stomach and intestines, pain may result from inflammation of the mucosa, ischemia, distention, or muscle spasm.
Somatic pain may arise from the skin (cutaneous) or from deeper structures such as bone or muscle, to be conducted by sensory nerves. Visceral pain originates in the organs and travels by sympathetic fibers. Depending on the cause, pain may be sudden and short-term, marked primarily by a reflex withdrawal. For example, if one touches a hot object, the hand is involuntarily jerked away from the source of injury. Or pain may be relatively continuous, as when infection or swelling is present.
Structures and Pain Pathways
Pain receptors or nociceptors are free sensory nerve endings that are present in most tissues of the body (Fig. 4-1). These sensory nerves may be stimulated by thermal, chemical, or physical means. Thermal means refer to extremes of temperature, mechanical means could refer to pressure, and chemical sources could include acids or compounds produced in the body, such as bradykinin, histamine, or prostaglandin.
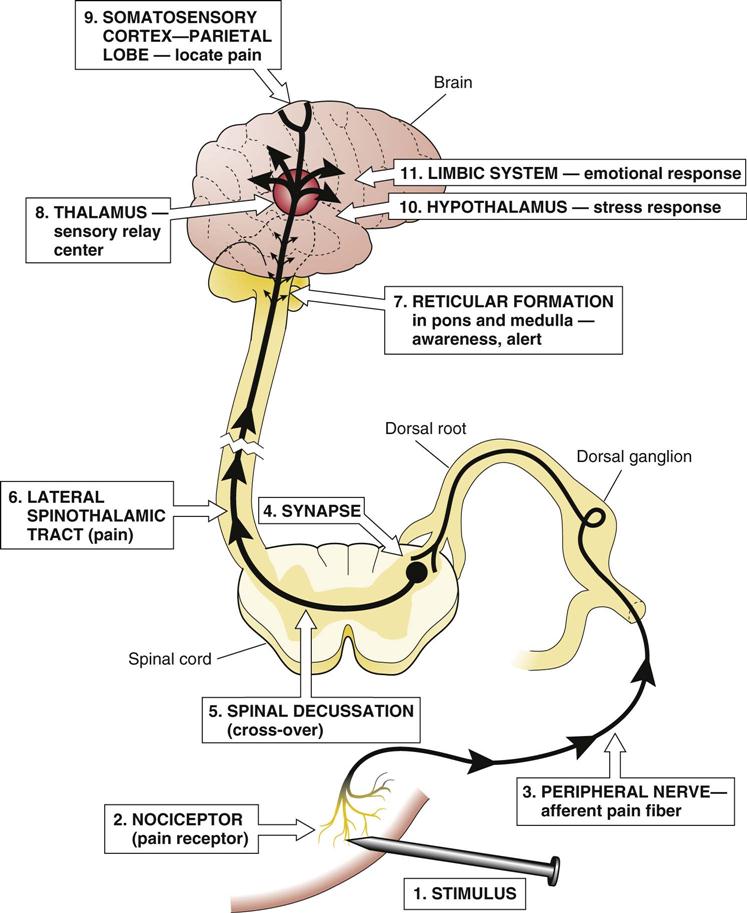
The pain threshold refers to the level of stimulation required to activate the nerve ending sufficiently for the individual to perceive pain. The associated nerve fibers then transmit the pain signal to the spinal cord and brain. The pain threshold is relatively constant over time and between individuals. The ability to withstand pain or the perception of its intensity is referred to as pain tolerance; this varies considerably with past pain experience and overall state of health.
Two types of afferent fibers conduct pain impulses: The myelinated A delta fibers that transmit impulses very rapidly and the unmyelinated C fibers that transmit impulses slowly. Acute pain—the sudden, sharp, localized pain related to thermal and physical stimuli primarily from skin and mucous membranes—is transmitted by the A delta fibers, whereas chronic pain—often experienced as a diffuse, dull, burning or aching sensation—is transmitted by C fibers. C fibers receive thermal, physical, and chemical stimuli from muscle, tendons, the myocardium, and the digestive tract as well as from the skin. The peripheral nerves transmit the afferent pain impulse to the dorsal root ganglia and then into the spinal cord through the dorsal horn or substantia gelatinosa (see Chapter 14).
Each spinal nerve conducts impulses from a specific area of the skin called a dermatome (see Fig. 14-22, which illustrates the areas of the skin innervated by each spinal nerve), and the somatosensory cortex is “mapped” to correspond to areas of the body so that the source of the pain can be interpreted in the brain (see Fig. 14-3 for a map of the brain). The dermatomes can be used to test for areas of sensory loss or pain sensation and thus determine the site of damage after spinal cord injuries.
At the spinal cord synapse, a reflex response to sudden pain results in a motor, or efferent, impulse back to the muscles that initiates an involuntary muscle contraction to move the body away from the source of pain. After the sensory impulse reaches the synapse, connecting neurons also transmit it across the spinal cord to the ascending tracts to the brain. There are two types of tracts in the spinothalamic bundle: The fast impulses for acute sharp pain travel in the neospinothalamic tract, whereas the slower impulses for chronic or dull pain use the paleospinothalamic tract. This double pathway explains the two stages of pain one often experiences with an injury to the skin, the initial sharp severe pain, followed by a duller but persistent throbbing or aching pain. These tracts connect with the reticular formation in the brain stem, hypothalamus, thalamus, and other structures as they ascend to the somatic sensory area in the cerebral cortex of the parietal lobe of the brain. It is here that the location and characteristics of the pain are perceived. The many branching connections from the ascending tracts provide information to other parts of the brain, forming the basis for an integrated response to pain.
The arousal state of the reticular activating system (RAS) in the reticular formation in the pons and medulla influences the brain’s awareness of the incoming pain stimuli. In clinical practice, many drugs depress the RAS, thereby decreasing the pain experienced. The hypothalamus plays a role in the response to pain through its connections with the pituitary gland and sympathetic nervous system. Response to pain usually involves a stress response (see Chapter 26) as well as an emotional response such as crying, moaning, or anger. There may be a physical response, perhaps rigidity, splinting, or guarding of an area of the body. The thalamus processes many types of sensory stimuli as they enter the brain and is important in the emotional response to pain through the limbic system.
Physiology of Pain and Pain Control
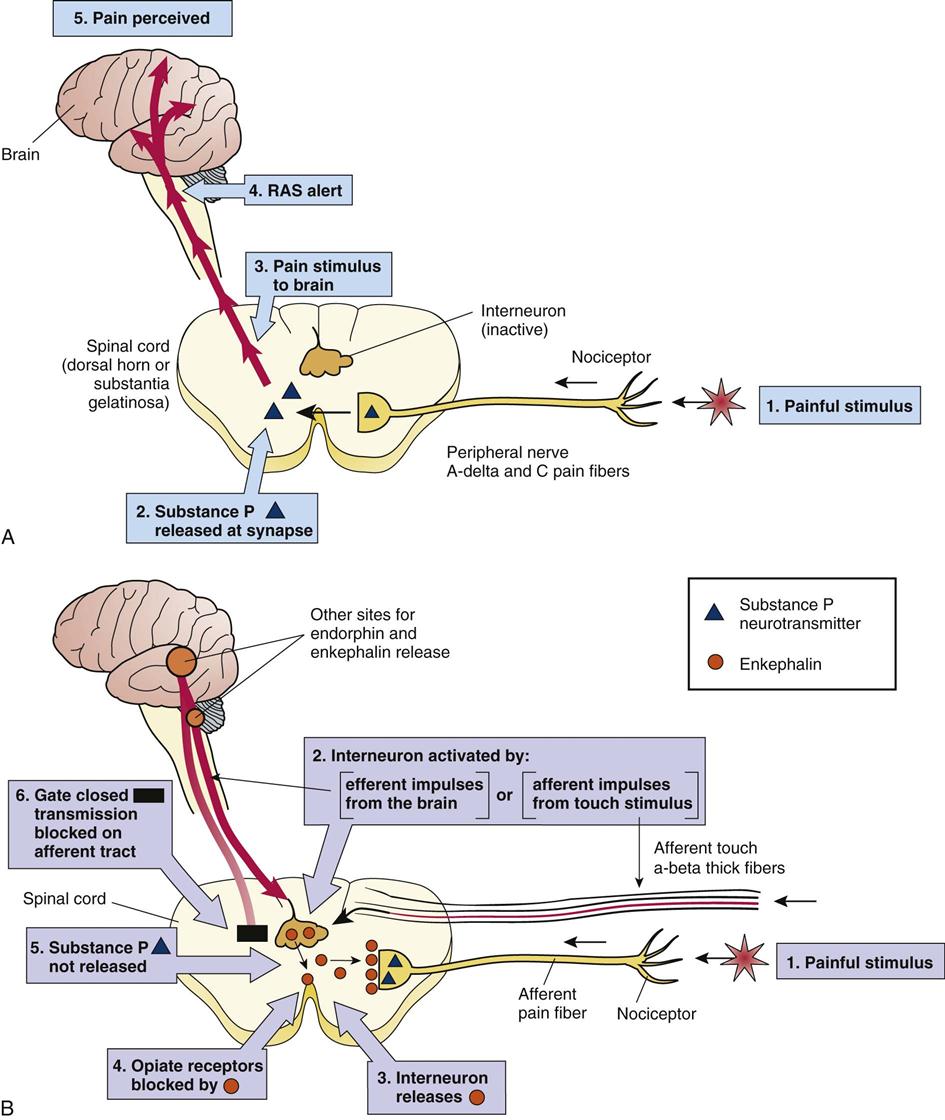
Pain is a highly complex phenomenon that is not fully understood. There are many variables in its source and perception and in the response to it in a specific individual. The gate-control theory has been modified as the complexity of pain is better realized, but the simple model serves as a useful tool and visual explanation of pain pathways that can be related to many concepts of pain and pain control. According to this theory, control systems, or “gates,” are built into the normal pain pathways in the body that can modify the entry of pain stimuli into the spinal cord and brain. These gates at the nerve synapses in the spinal cord and brain can be open, thus permitting the pain impulses to pass from the peripheral nerves to the spinothalamic tract and ascend to the brain (Fig. 4-2). Or they may be closed, reducing or modifying the passage of pain impulses. Gate closure can occur in response to other sensory stimuli along competing nerve pathways that may diminish the pain sensations or by modulating or inhibitory impulses from higher centers in the brain. For example, application of ice to a painful site may reduce pain because one is more aware of the cold than the pain. Transcutaneous electrical nerve stimulation (TENS) is a therapeutic intervention that increases sensory stimulation at a site, thus blocking pain transmission. Alternatively, the brain can inhibit or modify incoming pain stimuli by producing efferent or outgoing transmissions through the reticular formation. Many factors can activate this built-in control system, including prior conditioning, the emotional state of the affected person, or distraction by other events. This last phenomenon has been observed in many individuals who feel no pain when injured suddenly but do experience delayed onset of pain once they are no longer distracted by the immediate emergency situation.
The key to this analgesia system, or the blocking of pain impulses to the brain, is the release of a number of opiate-like chemicals (opioids) secreted by interneurons within the central nervous system. These substances block the conduction of pain impulses into the central nervous system. They resemble the drug morphine, which is derived from opium and is used as an analgesic, and are therefore called endorphins or endogenous morphine. Endorphins include enkephalins, dynorphins, and beta-lipotropins. Figure 4-2 illustrates how enkephalin is released in the spinal cord and is attached to opiate receptors on the afferent neuron, thus blocking release of the neurotransmitter substance P at the synapse. This process prevents transmission of the pain stimulus into the spinal cord. Serotonin is another chemical released in the spinal cord that acts on other neurons in the spinal cord to increase the release of enkephalins. Clients with clinical depression often report chronic pain due to reduction in serotonin levels in the brain. In addition, natural opiate receptors are found in many areas of the brain, as are secretions of endorphins, which can block pain impulses at that level. The body has its own endogenous analgesic or pain control system that explains some of the variables in pain perception and can be used to assist in pain control.
Characteristics of Pain
Signs and Symptoms
Pain is a real sensation but a subjective symptom perceived by each individual. There are many variations in the clinical picture of pain as well as the verbal reports of pain.
Possible details that may be helpful in diagnosing the severity and cause of pain include:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


